当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the human lipid exporter ABCB4 in a lipid environment.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41594-019-0354-3 Jeppe A Olsen 1, 2 , Amer Alam 1, 3 , Julia Kowal 1 , Bruno Stieger 4 , Kaspar P Locher 1
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41594-019-0354-3 Jeppe A Olsen 1, 2 , Amer Alam 1, 3 , Julia Kowal 1 , Bruno Stieger 4 , Kaspar P Locher 1
Affiliation
|
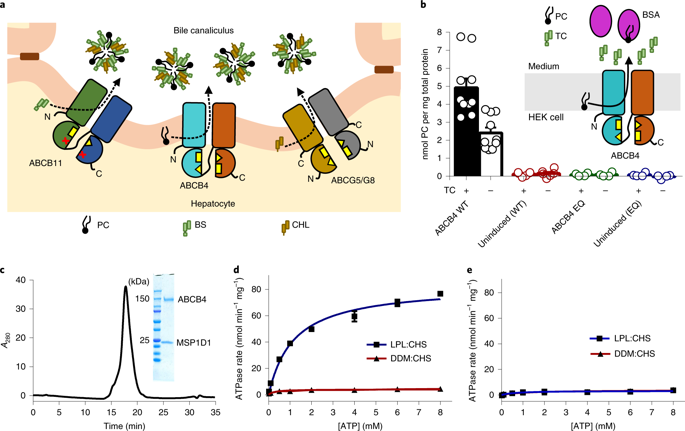
ABCB4 is an ATP-binding cassette transporter that extrudes phosphatidylcholine into the bile canaliculi of the liver. Its dysfunction or inhibition by drugs can cause severe, chronic liver disease or drug-induced liver injury. We determined the cryo-EM structure of nanodisc-reconstituted human ABCB4 trapped in an ATP-bound state at a resolution of 3.2 Å. The nucleotide binding domains form a closed conformation containing two bound ATP molecules, but only one of the ATPase sites contains bound Mg2+. The transmembrane domains adopt a collapsed conformation at the level of the lipid bilayer, but we observed a large, hydrophilic and fully occluded cavity at the level of the cytoplasmic membrane boundary, with no ligand bound. This indicates a state following substrate release but prior to ATP hydrolysis. Our results rationalize disease-causing mutations in human ABCB4 and suggest an 'alternating access' mechanism of lipid extrusion, distinct from the 'credit card swipe' model of other lipid transporters.
中文翻译:

脂质环境中人类脂质输出者 ABCB4 的结构。
ABCB4 是一种 ATP 结合盒转运蛋白,可将磷脂酰胆碱挤出到肝脏的胆小管中。其功能障碍或药物抑制可导致严重的慢性肝病或药物性肝损伤。我们确定了以 3.2 Å 的分辨率捕获在 ATP 结合状态中的纳米圆盘重组人 ABCB4 的冷冻电镜结构。核苷酸结合结构域形成包含两个结合的 ATP 分子的闭合构象,但只有一个 ATP 酶位点含有结合的 Mg2+。跨膜结构域在脂质双层水平采用塌陷构象,但我们在细胞质膜边界水平观察到一个大的、亲水的和完全封闭的空腔,没有配体结合。这表明在底物释放之后但在 ATP 水解之前的状态。
更新日期:2019-12-23
中文翻译:

脂质环境中人类脂质输出者 ABCB4 的结构。
ABCB4 是一种 ATP 结合盒转运蛋白,可将磷脂酰胆碱挤出到肝脏的胆小管中。其功能障碍或药物抑制可导致严重的慢性肝病或药物性肝损伤。我们确定了以 3.2 Å 的分辨率捕获在 ATP 结合状态中的纳米圆盘重组人 ABCB4 的冷冻电镜结构。核苷酸结合结构域形成包含两个结合的 ATP 分子的闭合构象,但只有一个 ATP 酶位点含有结合的 Mg2+。跨膜结构域在脂质双层水平采用塌陷构象,但我们在细胞质膜边界水平观察到一个大的、亲水的和完全封闭的空腔,没有配体结合。这表明在底物释放之后但在 ATP 水解之前的状态。









































 京公网安备 11010802027423号
京公网安备 11010802027423号