当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neonatal nonviral gene editing with the CRISPR/Cas9 system improves some cardiovascular, respiratory, and bone disease features of the mucopolysaccharidosis I phenotype in mice.
Gene Therapy ( IF 4.6 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41434-019-0113-4 Roselena Silvestri Schuh 1, 2 , Esteban Alberto Gonzalez 1, 3 , Angela Maria Vicente Tavares 1, 4 , Bruna Gazzi Seolin 4 , Lais de Souza Elias 1 , Luisa Natalia Pimentel Vera 1, 3 , Francyne Kubaski 3 , Edina Poletto 1, 3 , Roberto Giugliani 1, 3 , Helder Ferreira Teixeira 2 , Ursula Matte 1, 3 , Guilherme Baldo 1, 3, 4
Gene Therapy ( IF 4.6 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41434-019-0113-4 Roselena Silvestri Schuh 1, 2 , Esteban Alberto Gonzalez 1, 3 , Angela Maria Vicente Tavares 1, 4 , Bruna Gazzi Seolin 4 , Lais de Souza Elias 1 , Luisa Natalia Pimentel Vera 1, 3 , Francyne Kubaski 3 , Edina Poletto 1, 3 , Roberto Giugliani 1, 3 , Helder Ferreira Teixeira 2 , Ursula Matte 1, 3 , Guilherme Baldo 1, 3, 4
Affiliation
|
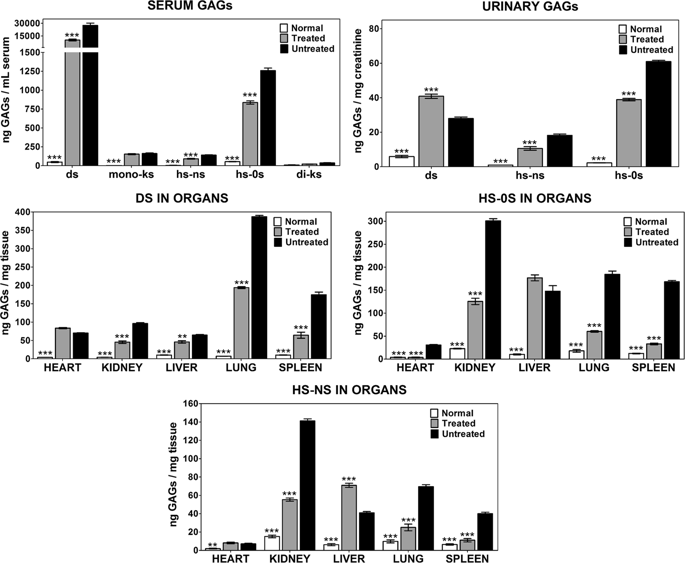
Mucopolysaccharidosis type I (MPS I) is caused by deficiency of alpha-L-iduronidase (IDUA), leading to multisystemic accumulation of glycosaminoglycans (GAG). Untreated MPS I patients may die in the first decades of life, mostly due to cardiovascular and respiratory complications. We previously reported that the treatment of newborn MPS I mice with intravenous administration of lipossomal CRISPR/Cas9 complexes carrying the murine Idua gene aiming at the ROSA26 locus resulted in long-lasting IDUA activity and GAG reduction in various tissues. Following this, the present study reports the effects of gene editing in cardiovascular, respiratory, bone, and neurologic functions in MPS I mice. Bone morphology, specifically the width of zygomatic and femoral bones, showed partial improvement. Although heart valves were still thickened, cardiac mass and aortic elastin breaks were reduced, with normalization of aortic diameter. Pulmonary resistance was normalized, suggesting improvement in respiratory function. In contrast, behavioral abnormalities and neuroinflammation still persisted, suggesting deterioration of the neurological functions. The set of results shows that gene editing performed in newborn animals improved some manifestations of the MPS I disorder in bone, respiratory, and cardiovascular systems. However, further studies will be imperative to find better delivery strategies to reach "hard-to-treat" tissues to ensure better systemic and neurological effects.
中文翻译:

利用CRISPR / Cas9系统进行的新生儿非病毒基因编辑可改善小鼠粘多糖贮积病I表型的某些心血管,呼吸和骨骼疾病特征。
I型粘多糖贮积病(MPS I)是由缺乏α-L-艾杜糖醛酸酶(IDUA)引起的,导致糖胺聚糖(GAG)的多系统积累。未经治疗的MPS I患者可能在生命的最初几十年内死亡,这主要是由于心血管和呼吸系统并发症所致。我们之前曾报道过,通过静脉注射携带针对ROSA26基因座的鼠Idua基因的脂质体CRISPR / Cas9复合物对新生MPS I小鼠进行治疗,可导致各种组织中IDUA活性持久和GAG降低。在此之后,本研究报告了基因编辑对MPS I小鼠的心血管,呼吸,骨骼和神经功能的影响。骨形态,特别是骨和股骨的宽度,显示出部分改善。尽管心脏瓣膜仍增厚,随着主动脉直径正常化,心脏质量和主动脉弹性蛋白断裂减少。肺阻力已恢复正常,表明呼吸功能得到改善。相反,行为异常和神经炎症仍持续存在,表明神经功能恶化。这组结果表明,在新生动物中进行的基因编辑改善了骨骼,呼吸系统和心血管系统中MPS I障碍的某些表现。然而,必须进行进一步的研究以找到更好的递送策略,以达到“难以治疗”的组织,以确保更好的全身和神经功能。行为异常和神经炎症仍持续存在,提示神经功能恶化。这组结果表明,在新生动物中进行的基因编辑改善了骨骼,呼吸系统和心血管系统中MPS I障碍的某些表现。然而,必须进行进一步的研究以找到更好的递送策略,以达到“难以治疗”的组织,以确保更好的全身和神经功能。行为异常和神经炎症仍持续存在,提示神经功能恶化。这组结果表明,在新生动物中进行的基因编辑改善了骨骼,呼吸系统和心血管系统中MPS I障碍的某些表现。然而,必须进行进一步的研究以找到更好的递送策略,以达到“难以治疗”的组织,以确保更好的全身和神经功能。
更新日期:2019-12-11
中文翻译:

利用CRISPR / Cas9系统进行的新生儿非病毒基因编辑可改善小鼠粘多糖贮积病I表型的某些心血管,呼吸和骨骼疾病特征。
I型粘多糖贮积病(MPS I)是由缺乏α-L-艾杜糖醛酸酶(IDUA)引起的,导致糖胺聚糖(GAG)的多系统积累。未经治疗的MPS I患者可能在生命的最初几十年内死亡,这主要是由于心血管和呼吸系统并发症所致。我们之前曾报道过,通过静脉注射携带针对ROSA26基因座的鼠Idua基因的脂质体CRISPR / Cas9复合物对新生MPS I小鼠进行治疗,可导致各种组织中IDUA活性持久和GAG降低。在此之后,本研究报告了基因编辑对MPS I小鼠的心血管,呼吸,骨骼和神经功能的影响。骨形态,特别是骨和股骨的宽度,显示出部分改善。尽管心脏瓣膜仍增厚,随着主动脉直径正常化,心脏质量和主动脉弹性蛋白断裂减少。肺阻力已恢复正常,表明呼吸功能得到改善。相反,行为异常和神经炎症仍持续存在,表明神经功能恶化。这组结果表明,在新生动物中进行的基因编辑改善了骨骼,呼吸系统和心血管系统中MPS I障碍的某些表现。然而,必须进行进一步的研究以找到更好的递送策略,以达到“难以治疗”的组织,以确保更好的全身和神经功能。行为异常和神经炎症仍持续存在,提示神经功能恶化。这组结果表明,在新生动物中进行的基因编辑改善了骨骼,呼吸系统和心血管系统中MPS I障碍的某些表现。然而,必须进行进一步的研究以找到更好的递送策略,以达到“难以治疗”的组织,以确保更好的全身和神经功能。行为异常和神经炎症仍持续存在,提示神经功能恶化。这组结果表明,在新生动物中进行的基因编辑改善了骨骼,呼吸系统和心血管系统中MPS I障碍的某些表现。然而,必须进行进一步的研究以找到更好的递送策略,以达到“难以治疗”的组织,以确保更好的全身和神经功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号