当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41594-019-0339-2 Kyle Tucker 1 , Eunyong Park 1, 2
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41594-019-0339-2 Kyle Tucker 1 , Eunyong Park 1, 2
Affiliation
|
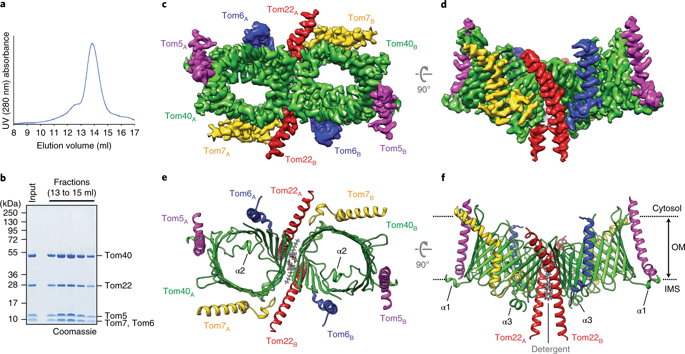
Nearly all mitochondrial proteins are encoded by the nuclear genome and imported into mitochondria after synthesis on cytosolic ribosomes. These precursor proteins are translocated into mitochondria by the TOM complex, a protein-conducting channel in the mitochondrial outer membrane. We have determined high-resolution cryo-EM structures of the core TOM complex from Saccharomyces cerevisiae in dimeric and tetrameric forms. Dimeric TOM consists of two copies each of five proteins arranged in two-fold symmetry: pore-forming β-barrel protein Tom40 and four auxiliary α-helical transmembrane proteins. The pore of each Tom40 has an overall negatively charged inner surface attributed to multiple functionally important acidic patches. The tetrameric complex is essentially a dimer of dimeric TOM, which may be capable of forming higher-order oligomers. Our study reveals the detailed molecular organization of the TOM complex and provides new insights about the mechanism of protein translocation into mitochondria.
中文翻译:

近原子分辨率下线粒体蛋白输入通道 TOM 复合物的冷冻电镜结构。
几乎所有的线粒体蛋白都由核基因组编码,并在胞质核糖体上合成后输入线粒体。这些前体蛋白通过 TOM 复合物(线粒体外膜中的一种蛋白质传导通道)转运到线粒体中。我们已经确定了来自酿酒酵母的二聚体和四聚体形式的核心 TOM 复合物的高分辨率冷冻电镜结构。二聚体 TOM 由 5 种蛋白质中的每一种以双重对称排列的两个拷贝组成:成孔 β-桶蛋白 Tom40 和四种辅助 α-螺旋跨膜蛋白。每个 Tom40 的孔都有一个整体带负电荷的内表面,这归因于多个具有重要功能的酸性斑块。四聚体复合物本质上是二聚体 TOM 的二聚体,它可能能够形成更高阶的寡聚体。
更新日期:2019-11-18
中文翻译:

近原子分辨率下线粒体蛋白输入通道 TOM 复合物的冷冻电镜结构。
几乎所有的线粒体蛋白都由核基因组编码,并在胞质核糖体上合成后输入线粒体。这些前体蛋白通过 TOM 复合物(线粒体外膜中的一种蛋白质传导通道)转运到线粒体中。我们已经确定了来自酿酒酵母的二聚体和四聚体形式的核心 TOM 复合物的高分辨率冷冻电镜结构。二聚体 TOM 由 5 种蛋白质中的每一种以双重对称排列的两个拷贝组成:成孔 β-桶蛋白 Tom40 和四种辅助 α-螺旋跨膜蛋白。每个 Tom40 的孔都有一个整体带负电荷的内表面,这归因于多个具有重要功能的酸性斑块。四聚体复合物本质上是二聚体 TOM 的二聚体,它可能能够形成更高阶的寡聚体。







































 京公网安备 11010802027423号
京公网安备 11010802027423号