当前位置:
X-MOL 学术
›
Modern Pathol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Programmed death-ligand 1 expression influenced by tissue sample size. Scoring based on tissue microarrays' and cross-validation with resections, in patients with, stage I-III, non-small cell lung carcinoma of the European Thoracic Oncology Platform Lungscape cohort.
Modern Pathology ( IF 7.1 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41379-019-0383-9 Erik Thunnissen 1 , Keith M Kerr 2 , Urania Dafni 3 , Lukas Bubendorf 4 , Stephen P Finn 5 , Alex Soltermann 6 , Wojciech Biernat 7 , Richard Cheney 8 , Erik Verbeken 9 , Arne Warth 10, 11 , Antonio Marchetti 12 , Ernst-Jan M Speel 13 , Saraswati Pokharel 14 , Anne Marie Quinn 15 , Kim Monkhorst 16 , Atilio Navarro 17 , Line Bille Madsen 18 , Zoi Tsourti 19 , Thomas Geiger 20 , Roswitha Kammler 20 , Solange Peters 21 , Rolf A Stahel 22 ,
Modern Pathology ( IF 7.1 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41379-019-0383-9 Erik Thunnissen 1 , Keith M Kerr 2 , Urania Dafni 3 , Lukas Bubendorf 4 , Stephen P Finn 5 , Alex Soltermann 6 , Wojciech Biernat 7 , Richard Cheney 8 , Erik Verbeken 9 , Arne Warth 10, 11 , Antonio Marchetti 12 , Ernst-Jan M Speel 13 , Saraswati Pokharel 14 , Anne Marie Quinn 15 , Kim Monkhorst 16 , Atilio Navarro 17 , Line Bille Madsen 18 , Zoi Tsourti 19 , Thomas Geiger 20 , Roswitha Kammler 20 , Solange Peters 21 , Rolf A Stahel 22 ,
Affiliation
|
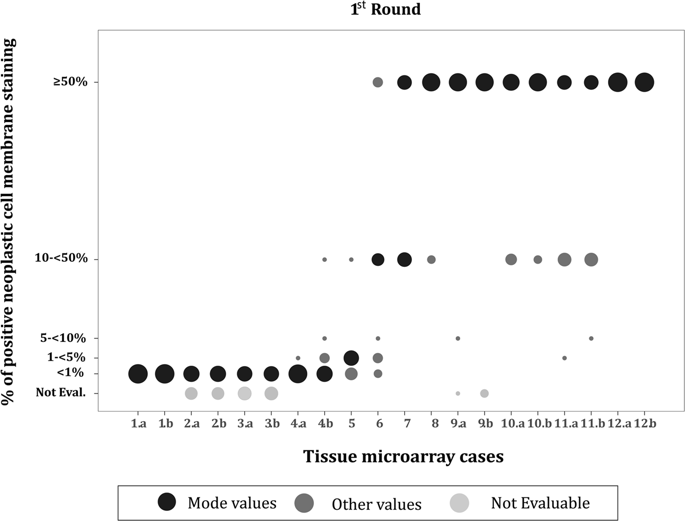
PD-L1, as assessed by immunohistochemistry, is a predictive biomarker for immuno-oncology treatment in lung cancer. Different scoring methods have been used to assess its status, resulting in a wide range of positivity rates. We use the European Thoracic Oncology Platform Lungscape non-small cell lung carcinoma cohort to explore this issue. PD-L1 expression was assessed via immunohistochemistry on tissue microarrays (up to four cores per case), using the DAKO 28-8 immunohistochemistry assay, following a two-round external quality assessment procedure. All samples were analyzed under the same protocol. Cross-validation of scoring between tissue microarray and whole sections was performed in 10% randomly selected samples. Cutoff points considered: ≥1, 50 (primarily), and 25%. At the two external quality assessment rounds, tissue microarray scoring agreement rates between pathologists were: 73% and 81%. There were 2008 cases with valid immunohistochemistry tissue microarray results (50% all cores evaluable). Concordant cases at 1, 25, and 50% were: 85, 91, and 93%. Tissue microarray core results were identical for 70% of cases. Sensitivity of the tissue microarray method for 1, 25, and 50% was: 80, 78, and 79% (specificity: 90, 95, 98%). Complete agreement between tissue microarrays and whole sections was achieved for 60% of the cases. Highest sensitivity rates for 1% and 50% cutoffs were detected for higher number of cores. Underestimation of PD-L1 expression on small samples is more common than overestimation. We demonstrated that classification of PD-L1 on small biopsy samples does not represent the overall expression of PD-L1 in all non-small cell cancer carcinoma cases, although the majority of cases are 'correctly' classified. In future studies, sampling more and larger biopsies, recording the biopsy size and tumor load may permit further refinement, increasing predictive accuracy.
中文翻译:

程序性死亡配体 1 的表达受组织样本大小的影响。基于欧洲胸部肿瘤平台 Lungscape 队列 I-III 期非小细胞肺癌患者的组织微阵列评分和切除交叉验证。
通过免疫组织化学评估,PD-L1 是肺癌免疫肿瘤治疗的预测生物标志物。使用不同的评分方法来评估其状态,从而产生了广泛的阳性率。我们使用欧洲胸部肿瘤平台 Lungscape 非小细胞肺癌队列来探讨这个问题。在两轮外部质量评估程序之后,使用 DAKO 28-8 免疫组织化学测定,通过组织微阵列(每个病例最多四个核心)上的免疫组织化学评估 PD-L1 表达。所有样品均按照相同方案进行分析。在 10% 随机选择的样本中进行组织微阵列和整个切片之间评分的交叉验证。考虑的截止点:≥1、50(主要)和 25%。在两轮外部质量评估中,病理学家之间的组织微阵列评分一致率为:73% 和 81%。有 2008 例具有有效的免疫组织化学组织微阵列结果(50% 所有核心均可评估)。 1%、25% 和 50% 的一致病例分别为:85%、91% 和 93%。 70% 的病例组织微阵列核心结果相同。组织微阵列方法对于 1、25 和 50% 的灵敏度分别为:80、78 和 79%(特异性:90、95、98%)。 60% 的病例在组织微阵列和整个切片之间实现了完全一致。对于更多的核心,检测到 1% 和 50% 截止值的最高灵敏度。小样本上 PD-L1 表达的低估比高估更常见。我们证明,尽管大多数病例被“正确”分类,但小活检样本上的 PD-L1 分类并不代表所有非小细胞癌病例中 PD-L1 的总体表达。 在未来的研究中,对更多更大的活检进行采样,记录活检大小和肿瘤负荷可能会进一步细化,从而提高预测准确性。
更新日期:2019-11-18
中文翻译:

程序性死亡配体 1 的表达受组织样本大小的影响。基于欧洲胸部肿瘤平台 Lungscape 队列 I-III 期非小细胞肺癌患者的组织微阵列评分和切除交叉验证。
通过免疫组织化学评估,PD-L1 是肺癌免疫肿瘤治疗的预测生物标志物。使用不同的评分方法来评估其状态,从而产生了广泛的阳性率。我们使用欧洲胸部肿瘤平台 Lungscape 非小细胞肺癌队列来探讨这个问题。在两轮外部质量评估程序之后,使用 DAKO 28-8 免疫组织化学测定,通过组织微阵列(每个病例最多四个核心)上的免疫组织化学评估 PD-L1 表达。所有样品均按照相同方案进行分析。在 10% 随机选择的样本中进行组织微阵列和整个切片之间评分的交叉验证。考虑的截止点:≥1、50(主要)和 25%。在两轮外部质量评估中,病理学家之间的组织微阵列评分一致率为:73% 和 81%。有 2008 例具有有效的免疫组织化学组织微阵列结果(50% 所有核心均可评估)。 1%、25% 和 50% 的一致病例分别为:85%、91% 和 93%。 70% 的病例组织微阵列核心结果相同。组织微阵列方法对于 1、25 和 50% 的灵敏度分别为:80、78 和 79%(特异性:90、95、98%)。 60% 的病例在组织微阵列和整个切片之间实现了完全一致。对于更多的核心,检测到 1% 和 50% 截止值的最高灵敏度。小样本上 PD-L1 表达的低估比高估更常见。我们证明,尽管大多数病例被“正确”分类,但小活检样本上的 PD-L1 分类并不代表所有非小细胞癌病例中 PD-L1 的总体表达。 在未来的研究中,对更多更大的活检进行采样,记录活检大小和肿瘤负荷可能会进一步细化,从而提高预测准确性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号