当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cdr1p highlights the role of the non-hydrolytic ATP-binding site in driving drug translocation in asymmetric ABC pumps.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.bbamem.2019.183131 Atanu Banerjee 1 , Alexis Moreno 2 , Mohammad Firoz Khan 3 , Remya Nair 4 , Suman Sharma 4 , Sobhan Sen 3 , Alok Kumar Mondal 5 , Jorgaq Pata 2 , Cédric Orelle 6 , Pierre Falson 6 , Rajendra Prasad 7
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.bbamem.2019.183131 Atanu Banerjee 1 , Alexis Moreno 2 , Mohammad Firoz Khan 3 , Remya Nair 4 , Suman Sharma 4 , Sobhan Sen 3 , Alok Kumar Mondal 5 , Jorgaq Pata 2 , Cédric Orelle 6 , Pierre Falson 6 , Rajendra Prasad 7
Affiliation
|
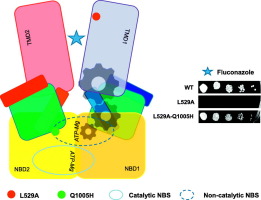
ATP-binding cassette (ABC) transporters couple ATP binding and hydrolysis to the translocation of allocrites across membranes. Two shared nucleotide-binding sites (NBS) participate in this cycle. In asymmetric ABC pumps, only one of them hydrolyzes ATP, and the functional role of the other remains unclear. Using a drug-based selection strategy on the transport-deficient mutant L529A in the transmembrane domain of the Candida albicans pump Cdr1p; we identified a spontaneous secondary mutation restoring drug-translocation. The compensatory mutation Q1005H was mapped 60 Å away, precisely in the ABC signature sequence of the non-hydrolytic NBS. The same was observed in the homolog Cdr2p. Both the mutant and suppressor proteins remained ATPase active, but remarkably, the single Q1005H mutant displayed a two-fold reduced ATPase activity and a two-fold increased drug-resistance as compared to the wild-type protein, pointing at a direct control of the non-hydrolytic NBS in substrate-translocation through ATP binding in asymmetric ABC pumps.
中文翻译:

Cdr1p强调了非水解ATP结合位点在驱动不对称ABC泵中的药物转运中的作用。
ATP结合盒(ABC)转运蛋白将ATP结合和水解作用与金属遍历膜的转运相结合。两个共享的核苷酸结合位点(NBS)参与此循环。在不对称的ABC泵中,只有一个水解ATP,而另一个的功能作用仍不清楚。对白色念珠菌Cdr1p跨膜结构域中运输缺陷型突变体L529A使用基于药物的选择策略;我们确定了自发的二级突变恢复药物易位。补偿性突变Q1005H被定位在60Å以外的位置,正好位于非水解NBS的ABC签名序列中。在同源物Cdr2p中观察到相同的结果。突变蛋白和抑制蛋白都保持ATPase活性,但值得注意的是,
更新日期:2019-11-18
中文翻译:

Cdr1p强调了非水解ATP结合位点在驱动不对称ABC泵中的药物转运中的作用。
ATP结合盒(ABC)转运蛋白将ATP结合和水解作用与金属遍历膜的转运相结合。两个共享的核苷酸结合位点(NBS)参与此循环。在不对称的ABC泵中,只有一个水解ATP,而另一个的功能作用仍不清楚。对白色念珠菌Cdr1p跨膜结构域中运输缺陷型突变体L529A使用基于药物的选择策略;我们确定了自发的二级突变恢复药物易位。补偿性突变Q1005H被定位在60Å以外的位置,正好位于非水解NBS的ABC签名序列中。在同源物Cdr2p中观察到相同的结果。突变蛋白和抑制蛋白都保持ATPase活性,但值得注意的是,











































 京公网安备 11010802027423号
京公网安备 11010802027423号