当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safe and neuroprotective vectors for long-term traumatic brain injury gene therapy.
Gene Therapy ( IF 4.6 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41434-019-0073-8 Daniela Blanco-Ocampo 1, 2 , Fabio Andrés Cawen 3 , Luis Angel Álamo-Pindado 1, 3 , María Luciana Negro-Demontel 1, 3 , Hugo Peluffo 1, 3
Gene Therapy ( IF 4.6 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41434-019-0073-8 Daniela Blanco-Ocampo 1, 2 , Fabio Andrés Cawen 3 , Luis Angel Álamo-Pindado 1, 3 , María Luciana Negro-Demontel 1, 3 , Hugo Peluffo 1, 3
Affiliation
|
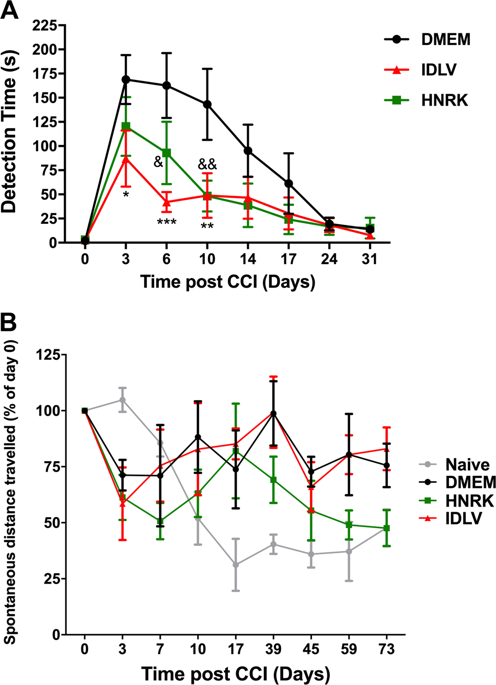
Traumatic brain injury (TBI) is a complex and progressive brain injury with no approved treatments that needs both short- and long-term therapeutic strategies to cope with the variety of physiopathological mechanisms involved. In particular, neuroinflammation is a key process modulating TBI outcome, and the potentiation of these mechanisms by pro-inflammatory gene therapy vectors could contribute to the injury progression. Here, we evaluate in the controlled cortical impact model of TBI, the safety of integrative-deficient lentiviral vectors (IDLVs) or the non-viral HNRK recombinant modular protein/DNA nanovector. These two promising vectors display different tropisms, transduction efficiencies, short- or long-term transduction or inflammatory activation profile. We show that the brain intraparenchymal injection of these vectors overexpressing green fluorescent protein after a CCI is not neurotoxic, and interestingly, can decrease the short-term sensory neurological deficits, and diminish the brain tissue loss at 90 days post lesion (dpl). Moreover, only IDLVs were able to mitigate the memory deficits elicited by a CCI. These vectors did not alter the microglial or astroglial reactivity at 90 dpl, suggesting that they do not potentiate the on-going neuroinflammation. Taken together, these data suggest that both types of vectors could be interesting tools for the design of gene therapy strategies targeting immediate or long-term neuropathological mechanisms of TBI.
中文翻译:

长期创伤性脑损伤基因治疗的安全和神经保护载体。
颅脑外伤(TBI)是一种复杂的进行性脑损伤,未经批准的治疗方法需要短期和长期的治疗策略来应对所涉及的各种生理病理机制。特别地,神经炎症是调节TBI结果的关键过程,而促炎基因治疗载体对这些机制的增强作用可能有助于损伤进展。在这里,我们评估在TBI的皮质控制冲击模型中,整合缺陷型慢病毒载体(IDLV)或非病毒HNRK重组模块蛋白/ DNA纳米载体的安全性。这两个有希望的载体显示出不同的向性,转导效率,短期或长期转导或炎性激活特征。我们显示,脑内实质注射这些载体后CCI过表达绿色荧光蛋白是无神经毒性的,有趣的是,可以减少短期感觉神经功能缺损,并减少病变后90天(dpl)的脑组织损失。此外,只有IDLV才能减轻CCI引起的内存不足。这些载体在90 dpl时没有改变小胶质细胞或星形胶质细胞的反应性,表明它们不能增强正在进行的神经炎症。综上所述,这些数据表明,两种类型的载体都可能是用于设计针对TBI的近期或长期神经病理学机制的基因治疗策略的有趣工具。并减少病变后90天(dpl)的脑组织损失。此外,只有IDLV才能减轻CCI引起的内存不足。这些载体在90 dpl时没有改变小胶质细胞或星形胶质细胞的反应性,表明它们不能增强正在进行的神经炎症。综上所述,这些数据表明,两种类型的载体都可能是设计针对TBI近期或长期神经病理机制的基因治疗策略的有趣工具。并减少病变后90天(dpl)的脑组织损失。此外,只有IDLV能够减轻CCI引起的内存不足。这些载体在90 dpl时没有改变小胶质细胞或星形胶质细胞的反应性,表明它们不能增强正在进行的神经炎症。综上所述,这些数据表明,两种类型的载体都可能是用于设计针对TBI的近期或长期神经病理学机制的基因治疗策略的有趣工具。
更新日期:2019-11-18
中文翻译:

长期创伤性脑损伤基因治疗的安全和神经保护载体。
颅脑外伤(TBI)是一种复杂的进行性脑损伤,未经批准的治疗方法需要短期和长期的治疗策略来应对所涉及的各种生理病理机制。特别地,神经炎症是调节TBI结果的关键过程,而促炎基因治疗载体对这些机制的增强作用可能有助于损伤进展。在这里,我们评估在TBI的皮质控制冲击模型中,整合缺陷型慢病毒载体(IDLV)或非病毒HNRK重组模块蛋白/ DNA纳米载体的安全性。这两个有希望的载体显示出不同的向性,转导效率,短期或长期转导或炎性激活特征。我们显示,脑内实质注射这些载体后CCI过表达绿色荧光蛋白是无神经毒性的,有趣的是,可以减少短期感觉神经功能缺损,并减少病变后90天(dpl)的脑组织损失。此外,只有IDLV才能减轻CCI引起的内存不足。这些载体在90 dpl时没有改变小胶质细胞或星形胶质细胞的反应性,表明它们不能增强正在进行的神经炎症。综上所述,这些数据表明,两种类型的载体都可能是用于设计针对TBI的近期或长期神经病理学机制的基因治疗策略的有趣工具。并减少病变后90天(dpl)的脑组织损失。此外,只有IDLV才能减轻CCI引起的内存不足。这些载体在90 dpl时没有改变小胶质细胞或星形胶质细胞的反应性,表明它们不能增强正在进行的神经炎症。综上所述,这些数据表明,两种类型的载体都可能是设计针对TBI近期或长期神经病理机制的基因治疗策略的有趣工具。并减少病变后90天(dpl)的脑组织损失。此外,只有IDLV能够减轻CCI引起的内存不足。这些载体在90 dpl时没有改变小胶质细胞或星形胶质细胞的反应性,表明它们不能增强正在进行的神经炎症。综上所述,这些数据表明,两种类型的载体都可能是用于设计针对TBI的近期或长期神经病理学机制的基因治疗策略的有趣工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号