Structure ( IF 4.4 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.str.2022.06.006 Inayathulla Mohammed 1 , Kai A Schmitz 1 , Niko Schenck 1 , Dimitrios Balasopoulos 1 , Annika Topitsch 1 , Timm Maier 1 , Jan Pieter Abrahams 2
|
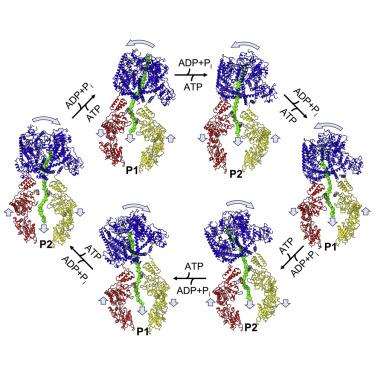
The mitochondrial Lon protease (LonP1) regulates mitochondrial health by removing redundant proteins from the mitochondrial matrix. We determined LonP1 in eight nucleotide-dependent conformational states by cryoelectron microscopy (cryo-EM). The flexible assembly of N-terminal domains had 3-fold symmetry, and its orientation depended on the conformational state. We show that a conserved structural motif around T803 with a high similarity to the trypsin catalytic triad is essential for proteolysis. We show that LonP1 is not regulated by redox potential, despite the presence of two conserved cysteines at disulfide-bonding distance in its unfoldase core. Our data indicate how sequential ATP hydrolysis controls substrate protein translocation in a 6-fold binding change mechanism. Substrate protein translocation, rather than ATP hydrolysis, is a rate-limiting step, suggesting that LonP1 is a Brownian ratchet with ATP hydrolysis preventing translocation reversal. 3-fold rocking motions of the flexible N-domain assembly may assist thermal unfolding of the substrate protein.
中文翻译:

人线粒体Lon蛋白酶的催化循环
线粒体 Lon 蛋白酶 (LonP1) 通过从线粒体基质中去除多余的蛋白质来调节线粒体健康。我们通过低温电子显微镜(cryo-EM)确定了八种核苷酸依赖性构象状态的 LonP1。N端域的柔性组装具有3重对称性,其方向取决于构象状态。我们表明,与胰蛋白酶催化三联体高度相似的 T803 周围的保守结构基序对于蛋白水解至关重要。我们表明 LonP1 不受氧化还原电位的调节,尽管在其展开酶核心的二硫键距离处存在两个保守的半胱氨酸。我们的数据表明连续 ATP 水解如何以 6 倍结合变化机制控制底物蛋白易位。底物蛋白质易位,而不是 ATP 水解,是一个限速步骤,表明 LonP1 是具有 ATP 水解防止易位逆转的布朗棘轮。柔性 N 域组件的 3 倍摇摆运动可能有助于底物蛋白的热解折叠。











































 京公网安备 11010802027423号
京公网安备 11010802027423号