Pediatric Drugs ( IF 3.4 ) Pub Date : 2022-06-13 , DOI: 10.1007/s40272-022-00507-0 Nina Magnolo 1 , Külli Kingo 2 , Vivian Laquer 3 , John Browning 4 , Adam Reich 5 , Jacek C Szepietowski 6 , Deborah Keefe 7 , Philemon Papanastasiou 8 , Weibin Bao 7 , Pascal Forrer 8 , Manmath Patekar 8
|
Background
The efficacy and safety of biologic treatments for children and adolescents with moderate to severe psoriasis should be examined over a considerable time period and in different subgroups.
Objective
We report the efficacy and safety of secukinumab low dose (LD) and high dose (HD) regimens in pediatric patients with moderate to severe psoriasis for up to Week 52.
Methods
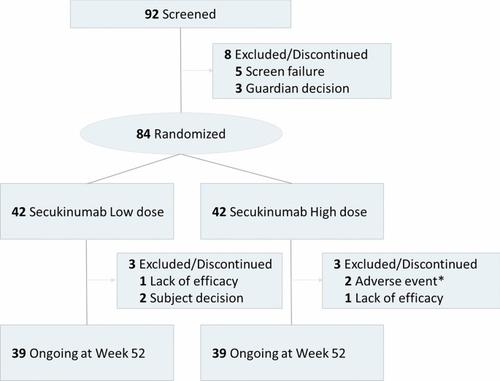
This was a randomized, open-label, parallel-group, multicenter study in patients aged 6 to < 18 years. Patients were randomized in a 1:1 ratio to receive LD (75/75/150 mg; N = 42) or HD (75/150/300 mg; N = 42) subcutaneous secukinumab. At randomization, patients were stratified by weight (< 25, 25 to < 50, ≥ 50 kg) and disease severity (moderate/severe). The study is ongoing; the present analysis included data up to Week 52 collected from August 29, 2018 (first patient first visit) to May 28, 2020 (last patient last visit for Week 52). Efficacy was measured using Investigator’s Global Assessment modified 2011 0/1 (IGA 0/1) and Psoriasis Area Severity Index (PASI) 75/90/100 response. Safety outcomes included assessment of adverse events.
Results
Of the 84 enrolled patients, 78 (92.9%) completed 52 weeks of treatment. Overall, response rates for PASI 75 and IGA 0/1 were similar between the LD (92.8/88.9%) and HD (93.3/84.7%) groups at Week 52. In the LD and HD groups, PASI 90/100 responses at Week 52 were 78.7/53.5% and 84.7/70.0%, respectively. The proportions of IGA 0/1 and PASI 75/90 responders were comparable for the age, body weight, and disease severity subgroups in the secukinumab LD and HD groups. Mean absolute PASI change from baseline at week 52 was − 17.3 ± standard deviation 5.0 and − 18.2 ± 7.0, a percentage change of − 94.3 and − 94.5% for the LD and HD groups, respectively. More than 70% of evaluable patients achieved Children’s Dermatology Life Quality Index 0/1 at Week 52 (LD 70.7%; HD 70.3%). The safety profile was consistent with that in adults, with no new safety signals for either secukinumab dosing regimen.
Conclusion
A high proportion of pediatric patients with psoriasis responded to both dosing regimens of secukinumab and maintained clinical responses through 52 weeks of treatment. No clinical difference was observed in the efficacy of secukinumab across the pediatric subgroups. Safety events were consistent with the established safety profile of secukinumab.
Trial Registration
ClinicalTrials.gov: NCT03668613.
中文翻译:

苏金单抗在中度至重度斑块状银屑病儿科患者中的跨亚组疗效和总体安全性:III 期随机研究的第 52 周结果
背景
生物治疗对中度至重度银屑病儿童和青少年的疗效和安全性应在相当长的一段时间内和不同的亚组中进行检查。
客观的
我们报告了苏金单抗低剂量 (LD) 和高剂量 (HD) 方案在中度至重度银屑病儿科患者中长达 52 周的疗效和安全性。
方法
这是一项针对 6 至 <18 岁患者的随机、开放标签、平行组、多中心研究。患者以 1:1 的比例随机接受 LD(75/75/150 mg;N = 42)或 HD(75/150/300 mg;N = 42)皮下苏金单抗。在随机化时,患者按体重(< 25、25 至 < 50、≥ 50 kg)和疾病严重程度(中度/重度)进行分层。该研究正在进行中;目前的分析包括从 2018 年 8 月 29 日(第一位患者首次就诊)到 2020 年 5 月 28 日(最后一位患者最后一次就诊为第 52 周)收集的截至第 52 周的数据。使用 Investigator's Global Assessment 修改 2011 0/1 (IGA 0/1) 和银屑病区域严重性指数 (PASI) 75/90/100 响应来测量疗效。安全性结果包括对不良事件的评估。
结果
在 84 名入组患者中,78 名(92.9%)完成了 52 周的治疗。总体而言,第 52 周时,LD (92.8/88.9%) 和 HD (93.3/84.7%) 组的 PASI 75 和 IGA 0/1 响应率相似。在 LD 和 HD 组中,PASI 90/100 在第 52 周的响应率52 分别为 78.7/53.5% 和 84.7/70.0%。在苏金单抗 LD 和 HD 组中,IGA 0/1 和 PASI 75/90 反应者的比例在年龄、体重和疾病严重程度亚组中具有可比性。在第 52 周,平均绝对 PASI 变化为 - 17.3 ± 标准差 5.0 和 - 18.2 ± 7.0,LD 和 HD 组的百分比变化分别为 - 94.3 和 - 94.5%。超过 70% 的可评估患者在第 52 周达到儿童皮肤病学生活质量指数 0/1(LD 70.7%;HD 70.3%)。安全性与成人一致,
结论
大部分患有银屑病的儿科患者对苏金单抗的两种给药方案都有反应,并在 52 周的治疗中保持临床反应。苏金单抗的疗效在儿科亚组中未观察到临床差异。安全事件与已确立的苏金单抗安全性特征一致。
试用注册
ClinicalTrials.gov:NCT03668613。











































 京公网安备 11010802027423号
京公网安备 11010802027423号