Abstract
Background
The efficacy and safety of biologic treatments for children and adolescents with moderate to severe psoriasis should be examined over a considerable time period and in different subgroups.
Objective
We report the efficacy and safety of secukinumab low dose (LD) and high dose (HD) regimens in pediatric patients with moderate to severe psoriasis for up to Week 52.
Methods
This was a randomized, open-label, parallel-group, multicenter study in patients aged 6 to < 18 years. Patients were randomized in a 1:1 ratio to receive LD (75/75/150 mg; N = 42) or HD (75/150/300 mg; N = 42) subcutaneous secukinumab. At randomization, patients were stratified by weight (< 25, 25 to < 50, ≥ 50 kg) and disease severity (moderate/severe). The study is ongoing; the present analysis included data up to Week 52 collected from August 29, 2018 (first patient first visit) to May 28, 2020 (last patient last visit for Week 52). Efficacy was measured using Investigator’s Global Assessment modified 2011 0/1 (IGA 0/1) and Psoriasis Area Severity Index (PASI) 75/90/100 response. Safety outcomes included assessment of adverse events.
Results
Of the 84 enrolled patients, 78 (92.9%) completed 52 weeks of treatment. Overall, response rates for PASI 75 and IGA 0/1 were similar between the LD (92.8/88.9%) and HD (93.3/84.7%) groups at Week 52. In the LD and HD groups, PASI 90/100 responses at Week 52 were 78.7/53.5% and 84.7/70.0%, respectively. The proportions of IGA 0/1 and PASI 75/90 responders were comparable for the age, body weight, and disease severity subgroups in the secukinumab LD and HD groups. Mean absolute PASI change from baseline at week 52 was − 17.3 ± standard deviation 5.0 and − 18.2 ± 7.0, a percentage change of − 94.3 and − 94.5% for the LD and HD groups, respectively. More than 70% of evaluable patients achieved Children’s Dermatology Life Quality Index 0/1 at Week 52 (LD 70.7%; HD 70.3%). The safety profile was consistent with that in adults, with no new safety signals for either secukinumab dosing regimen.
Conclusion
A high proportion of pediatric patients with psoriasis responded to both dosing regimens of secukinumab and maintained clinical responses through 52 weeks of treatment. No clinical difference was observed in the efficacy of secukinumab across the pediatric subgroups. Safety events were consistent with the established safety profile of secukinumab.
Trial Registration
ClinicalTrials.gov: NCT03668613.
Similar content being viewed by others
The study examined the efficacy and safety of secukinumab after 52 weeks of treatment in children and adolescents with moderate to severe psoriasis. We present the efficacy of secukinumab by body weight, disease severity, and dose groups. In children and adolescents with moderate to severe psoriasis, the clinical benefit of secukinumab observed during the first 24 weeks of treatment was maintained through 52 weeks. In addition, the efficacy of secukinumab for skin clearance was consistent across weight and age subgroups. |
Secukinumab was generally well tolerated in children and adolescents after 52 weeks of treatment, with no new safety concerns observed. The safety profile of secukinumab was consistent with previous psoriasis clinical studies in adult patients. |
1 Introduction
Plaque psoriasis is the most common variant of psoriasis in pediatric patients. Its prevalence varies according to the study population (e.g., region-wide variation in prevalence) and is reported to be approximately 1% in children and adolescents [1, 2]. Plaque psoriasis in pediatric patients affects quality of life (QoL) and increases the risk of psychosocial disorders, depression, and anxiety [3, 4].
The European Medicines Agency (EMA) has approved etanercept, ixekizumab, and ustekinumab for plaque psoriasis in pediatric patients aged ≥ 6 years and adalimumab in pediatric patients aged ≥ 4 years. In addition, the US FDA has approved ixekizumab, etanercept, and ustekinumab [5] in children aged ≥ 6, ≥ 4, and ≥ 6 years, respectively [6]. Given the limited biologics licensed for psoriasis in pediatric patients to date, there is an unmet need for additional therapeutic choices that are safe, effective, and convenient [7].
Secukinumab is a fully human monoclonal antibody that selectively neutralizes interleukin-17A, a cornerstone cytokine involved in the development of psoriasis. Secukinumab has demonstrated sustained long-term efficacy with a favorable safety profile in various psoriatic manifestations in adults [8,9,10,11]. Following the success of two pivotal phase III studies (ClinicalTrials.gov ID: NCT03668613 and NCT02471144), the EMA approved secukinumab in July 2020 in children and adolescents aged ≥ 6 years [12], and the FDA approved it in May 2021 in pediatric patients aged ≥ 6 years for treatment of moderate to severe plaque psoriasis [13]. The dose recommended for pediatric plaque psoriasis by the EU summary of product characteristics [12] and by the FDA [13] is based on body weight and is delivered by subcutaneous injection at weeks 0, 1, 2, 3, and 4, and then every 4 weeks thereafter. If the body weight is < 50 kg at the time of dosing, the recommended dose is 75 mg; if the body weight is ≥ 50 kg, the recommended dose is 150 mg. In Europe, for patients weighing ≥ 50 kg, the dose may be increased to 300 mg (as some patients may derive additional benefit from the higher dose).
The aforementioned second pivotal phase III trial (NCT03668613) assessed the efficacy and safety of two secukinumab dosage regimens in pediatric patients with moderate to severe plaque psoriasis for up to 24 weeks [14]. In addition to the evidence that secukinumab is effective in pediatric patients with plaque psoriasis, subgroup analyses were performed to evaluate and understand the influence of age, body weight, dose groups, and disease severity on the efficacy of secukinumab.
2 Methods
2.1 Study Design
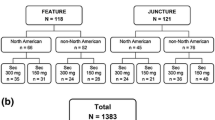
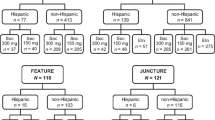
The study was conducted from August 29, 2018 (first patient first visit) to the cut-off date of May 28, 2020 (last patient, last visit for Week 52). This was an open-label, parallel-group, two-arm, multicenter study in pediatric patients aged 6 to < 18 years at randomization with moderate to severe chronic plaque psoriasis. The study consisted of three periods: screening (up to 4 weeks), treatment (of 208 weeks), and post-treatment follow-up (of 16 weeks) (Fig. 1 in the electronic supplementary material [ESM])). We report the study results for 52 weeks. At randomization, patients were stratified by body weight (< 25, 25 to < 50 and ≥ 50 kg) and disease severity (moderate or severe). As per protocol, it was planned that at least 60 patients with moderate psoriasis and at least five patients with a body weight < 25 kg would be enrolled.
Historical placebo control was obtained using data from qualifying studies (NCT02471144, NCT01365455, NCT01358578, NCT01555125, and NCT01636687) for the primary and key secondary endpoint analysis [14]. During discussions with Health Authorities, the US FDA and the EMA Pediatric Committee supported reducing placebo exposure and overall clinical study burden for the pediatric population by using the historical placebo extrapolation approach.
2.2 Participants
2.2.1 Inclusion and Exclusion Criteria
The eligibility criteria for this study have been described in detail [14]. Briefly, pediatric patients enrolled in this study were aged 6 to < 18 years and had a diagnosis of moderate to severe plaque psoriasis. Criteria for moderate to severe disease were a Psoriasis Area and Severity Index (PASI) score of ≥ 12, an Investigator’s Global Assessment modified 2011 (IGA mod 2011) score of ≥ 3, and body surface area (BSA) involvement of ≥ 10% at randomization. Patients included in this study had a history of plaque psoriasis ≥ 3 months before randomization and were eligible for systemic therapy. Exclusion criteria were described in the 24-week publication of the study [14].
2.3 Intervention
Patients were randomized using a 1:1 ratio at the randomization visit to two treatment arms (secukinumab low dose [LD] or high dose [HD]) and received a dose according to their weight category (< 25, 25 to < 50 and ≥ 50 kg): patients weighing < 25 kg received secukinumab 75 mg (both LD and HD groups), patients weighing 25 to < 50 kg received 75 mg (LD group) or 150 mg (HD group), and patients weighing ≥ 50 kg received 150 mg (LD group) or 300 mg (HD group).
2.4 Outcome Measures
The efficacy of secukinumab was evaluated by assessing PASI 75/90/100 and IGA mod 2011 0/1 response rates through Week 52. Secukinumab efficacy was also investigated across subgroups of pediatric patients defined by age (6 to < 12 and 12 to < 18 years), body weight, and disease severity. The disease severity was either moderate (PASI score 12 to < 20 and IGA 3/4 or PASI score ≥ 20 and IGA 3) or severe (PASI score ≥ 20 and IGA 4). We also assessed the impact of secukinumab on the QoL of patients by measuring Children’s Dermatology Life Quality Index (CDLQI) 0/1 response (last observation carried forward [LOCF]) and achievement of a CDLQI 0/1 over time, up to Week 52. Additionally, the clinical safety of secukinumab was assessed up to Week 52. Safety outcomes included assessment of adverse events (AEs) including treatment-emergent AEs (TEAEs), serious AEs (SAEs), AEs of special interest, laboratory tests, and vital signs.
2.5 Statistical Methods
A randomized set (all randomized patients) was used for patient disposition, demographic, and baseline disease characteristic summaries. A full analysis set was used for efficacy analyses that included all patients from the randomized set to whom study treatment had been assigned. The safety set was analyzed for safety summaries, which comprised all patients who received at least one dose of study treatment during the treatment period.
Summary statistics are presented for PASI 75, PASI 90, PASI 100, and IGA 0/1 responses by visit. In addition, summaries of the efficacy endpoints were presented by age, disease severity strata, and body weight by dose group. Confidence intervals for response rates were derived based on the exact method (for multiple imputation [MI] outputs). Summary statistics were provided for absolute PASI scores. Percentage change from baseline summaries for PASI scores by visit and treatment are reported.
For the safety analysis, TEAEs were summarized. TEAEs were defined as events that started after the first dose of study treatment and within 84 days after the last study treatment or as events present prior to the first dose of study treatment, but that increased in severity based on International Conference on Harmonisation Medical Dictionary for Regulatory Activities (MedDRA®) preferred term (PT) within 84 days after the last study treatment. AEs and SAEs were summarized by presenting the number and percentage of patients with least one AE and with an AE in each primary system organ class (SOC) and by each individual AE (PT). Summaries were also presented for AEs by severity and for study treatment-related AEs. Separate summaries were provided for death, SAEs, AEs leading to treatment discontinuation, and AEs leading to study drug interruption. MedDRA® version 23.0 was used to report the AEs. A Bayesian logistic-regression model was incorporated into the analysis of placebo response rates from historical data using a meta-analytic-predictive framework (using a Markov chain Monte-Carlo method in Stan, version 2.17.1). For PASI and IGA 0/1 response variables, missing values were imputed with MI regardless of the reason for missing data. Missing PASI, IGA, and CDLQI scores were imputed with LOCF.
3 Results
3.1 Participants
Of the 84 patients originally randomized to the secukinumab LD and HD groups, 78 (92.9%) patients completed the Week 52 visit. Six patients discontinued the study (three each in the LD and HD groups); reasons for discontinuations were AEs (two patients [2.4%] in the HD group: one because of alanine aminotransferase and aspartate aminotransferase elevation and one because of hemorrhagic diarrhea), lack of efficacy (one patient [2.4%] each in the LD and HD groups), and subject decision (two [2.4%] patients in the LD group) (Fig. 1). The baseline characteristics were balanced between the dose groups and have been reported [14] (Table 1 in the ESM). A history of psoriatic arthritis was noted in a patient in the secukinumab LD group at baseline. All randomized patients had previously received psoriasis therapies. A total of 42.9% of patients in both dose groups had received systemic therapies for psoriasis; however, the majority of patients in the LD (90.5%) and HD (85.7%) groups were naive to biologic treatment.
3.2 Efficacy
The study achieved its primary and key secondary endpoints (as previously shown in Magnolo et al. [14]). Early improvements were seen with secukinumab, with PASI 75 responses of 61.9% (LD) and 71.4% (HD) at Week 4. PASI 75/IGA 0/1 responses increased to Week 24 (LD: 95.2%/88.1% and HD: 96.9%/93.3%) and were sustained up to Week 52 (LD: 92.8%/88.9% and HD: 93.3%/84.7%). At Week 52, PASI 90/100 responses were 78.7%/53.5% in the LD group and 84.7%/70.0% in the HD group (Fig. 2). The response rates were calculated using the MI approach. The results based on nonresponder imputation were consistent with those based on the MI method. Mean absolute PASI change from baseline at 52 weeks was − 17.3 ± standard deviation 5.0 and − 18.2 ± 7.0, corresponding to a change of − 94.3% and − 94.5% for the LD and HD groups, respectively (Fig. 3). Overall, CDLQI 0/1 response was achieved in 50.0% and 61.9% of patients at Week 12, which increased to 70.7% and 70.3% at Week 52, in the LD and HD groups, respectively (Fig. 4).
PASI 75/90/100a and IGA mod 2011 0/1 responses up to Week 52. a Low-dose secukinumab (N = 42), b high-dose secukinumab (N = 42). Multiple imputation method was used to impute missing values. IGA mod 2011 0/1 Investigator’s Global Assessment modified 2011, N total number of patients, PASI 75/90/100 percentage of patients who achieved a ≥ 75%, ≥ 90%, or 100% reduction in Psoriasis Area and Severity Index score from baseline. aPASI 75/90/100, percentage of patients who achieved a ≥ 75%/≥ 90%/100% reduction in PASI score from BL Multiple imputation method was used to impute missing values
Percentage of patients with CDLQI 0/1a. Response through Week 52 (last observation carried forward; full analysis set). a0/1, no impairment in patient’s quality of life. CDLQI Children’s Dermatology Life Quality Index; m number of patients evaluable, n number of patients with response, SEC secukinumab
3.3 Subgroup Analyses
3.3.1 Age
In the 6 to < 12 years age group, PASI 75 and IGA 0/1 response rates were similar over time. The LD group achieved 100% response rate for PASI 75 from Week 12 and for IGA 0/1 from Week 16 until Week 52 (except for IGA 0/1 for one patient at Week 48). Overall, PASI 90/100 response rates from Week 16 to Week 24 were higher in the LD group than the HD group, which were comparable around Week 32 and toward Week 52; response rates were higher in the HD group.
In the 12 to < 18 years age group, PASI response rates (75/90/100) were higher in the HD group around Week 8 (PASI 90 responses were higher at Week 8 and Week 12), and responses thereafter were comparable until Week 52 for PASI 75/90. PASI 100 response rates were overall the same or comparable, except at Week 32 and Week 52, when rates were higher in the HD group. IGA 0/1 data were comparable over the 52-week period (Fig. 2 in the ESM).
3.3.2 Body Weight and Dose
In the < 25 kg subgroup, from Week 24 to Week 52, all eight patients (100%) were responders to IGA 0/1 and PASI 75/90; from Week 32, six of the eight (75%) patients were PASI 100 responders. In the 25 to < 50 kg subgroup, at the majority of assessment visits from Week 12 to Week 24, the IGA 0/1 and PASI 75/90/100 response rates were numerically higher in patients receiving the LD than in those receiving the HD. In the ≥ 50 kg weight subgroup, the IGA 0/1 and PASI 75 responses from Week 8 to Week 52 were comparable between the dose groups. At almost all assessment visits, the PASI 90/100 responses from Week 8 to Week 52 were numerically higher in the HD group than in the LD group (Fig. 3 in the ESM).
3.3.3 Disease Severity and Dose
Response rates for PASI 75 and IGA 0/1 from Week 12 were comparable over time across the two secukinumab doses in the moderate disease subgroup; PASI 90 response rates in the HD group were slightly higher than in the LD group from Week 32 to Week 52. Towards Week 52, PASI 100 response rates were consistently higher in the HD than in the LD group.
For patients with severe disease, the PASI 75 response rates were similar in the dose groups at Week 12 and from Week 40 to Week 52. At Week 52, the PASI 90/100 responses were similar across the dose groups in patients with severe disease, whereas the IGA 0/1 continued to be higher in the LD group than in the HD group (Fig. 4 in the ESM).
3.4 Safety
Overall, 65.5% (55/84) of patients reported TEAEs. The incidence of TEAEs was similar in the LD (28/42 patients [66.7%]) and HD (27/42 patients [64.3%]) groups (Table 1). The majority of AEs were mild (44/84 patients) in severity, and incidence rates were similar in the LD (23/42 patients) and the HD (21/42 patients) groups. The incidence of moderate AEs was the same in both secukinumab dosing groups (4/42 patients in each group). Treatment-emergent SAEs were reported in two patients in the LD group and one patient in the HD group (Table 1). The most commonly reported events in the SOCs were infections and infestations (40/84 patients; 47.6% overall), gastrointestinal disorders (12/84 patients; 14.3% overall), and skin and subcutaneous tissue disorders (12/84 patients; 14.3% overall). The AEs in the SOCs were mainly driven by nasopharyngitis (15/84 patients; 17.9% overall) (Table 1), diarrhea (3/84 patients; 3.6% overall) (data not shown), vomiting (3/84 patients; 3.6% overall) (data not shown), and acne (6/84 patients; 7.1% overall) (Table 1). The causality assessments indicated no link between either secukinumab dose groups and acne events in the pediatric patients in this study. All acne cases were mild in severity and did not lead to treatment discontinuation, interruption, or modification. Diarrhea (3/84 patients; 3.6% overall) and vomiting (3/84 patients; 3.6% overall) were major events in the SOC of gastrointestinal disorders; none of the events in this SOC were serious. Overall, causality of 10 of the 12 cases of gastrointestinal disorders were not attributed to secukinumab dose groups.
The most commonly reported AEs by PT were nasopharyngitis (LD: 9/42 patients [21.4%]; HD: 6/42 [14.3%]) and acne (LD: 5/42 patients [11.9%]; HD (1/42 patients [2.4%]). One patient from the LD group reported skin Candida, with two mild severity events related to skin Candida in the left armpit. The first incident occurred 27 days following the most recent secukinumab dose and was resolved in 11 days (days 278–288) without the need for secukinumab discontinuation or interruptions. The second event (reported on Day 330) occurred 22 days after the most recent dose of secukinumab and was ongoing at the time of this analysis; no adjustment in secukinumab dose was recommended. Another patient in the HD group reported vulvovaginal candidiasis; overall, this patient reported three mild events with durations of 2 (Day 9 and 10), 9 (Days 13–21), and 4 (Days 323–326) days. All these events resolved without any secukinumab discontinuation, interruption, or modification. Neutropenia or leukopenia were reported in five patients: three in the LD group and two in the HD group. The neutropenia and leukopenia events occurred prior to Week 24 and were reported previously [14]. No new events were reported after Week 24.
AEs related to the standard MedDRA query (SMQ) hypersensitivity were reported in three (7.1%) patients in the LD group and one patient (2.4%) in the HD group. The AEs included drug hypersensitivity and eczema in the LD group and erythema nodosum in the HD group. All these events were of mild severity and resolved completely in all subjects while still on treatment. These events were also considered unrelated to the study drug. In addition, none of these events (drug hypersensitivity, eczema, and erythema nodosum) were serious or led to study drug discontinuation/interruptions.
One potential case of inflammatory bowel disease showed nonserious hemorrhagic diarrhea that resolved and was followed by a noninflammatory diarrhea episode at the end of study visit. ‘Injection site reactions’ or ‘application and instillation site reactions’ (high-level terms) of mild intensity were observed in three patients across the dose groups; these events were of short duration and resolved without treatment prior to Week 52. No deaths were reported in the study up to Week 52. AEs leading to discontinuation were reported in two patients in the HD group (elevated alanine aminotransferase and aspartate aminotransferase levels of mild severity, and hemorrhagic diarrhea of mild severity) over 52 weeks. During the entire treatment, none of the patients in the study developed treatment-emergent antidrug antibodies (ADA). ADAs were detected in one patient at randomization and thus were not associationed with treatment.
4 Discussion
The current study in pediatric patients with moderate to severe chronic plaque psoriasis treated with secukinumab is ongoing; this article presents results up to Week 52. The study has achieved the primary objective and the key secondary objective: both secukinumab dosing regimens were superior to historical placebo, as previously reported [14]. Secukinumab treatment demonstrated significant improvements in PASI 75/90/100 and IGA 0/1 responses until Week 24 and the responses were maintained up to Week 52. In addition, the efficacy of secukinumab, as measured by PASI and IGA 0/1 response rates, was generally consistent across the different subgroups of pediatric patients. Apart from clinical efficacy, secukinumab treatment improved QoL as measured using the CDLQI in pediatric patients with psoriasis, with 70% of pediatric patients in both secukinumab dose groups indicating that psoriasis had no detrimental effect on their QoL by Week 52.
Clinical studies have demonstrated the sustained long-term efficacy of secukinumab in adult patients with moderate to severe chronic plaque psoriasis [11, 15]. The CLEAR and SCULPTURE studies reported PASI 75/90/100 responses in 92.4%/76.3%/45.8% and 88.9%/68.5%/43.8% of adults, respectively, receiving 300 mg secukinumab for moderate to severe chronic plaque psoriasis at Week 52 [11, 15]. In the current pediatric study, at Week 52, over 90% of patients achieved PASI 75 responses, more than 78% patients achieved PASI 90 responses, and over 53% of patients achieved PASI 100 responses with both secukinumab dosing regimens.
Bodemer et al. [16] demonstrated the efficacy and safety of both LD and HD secukinumab in pediatric patients with severe chronic plaque psoriasis over 52 weeks. At Week 52, PASI 75/90/100 and IGA0/1 responses in this study comprising pediatric patients with moderate to severe plaque psoriasis were in line with the responses in the earlier study comprising pediatric patients with severe chronic plaque psoriasis [16].
Our analysis also demonstrated that the efficacy of secukinumab was overall comparable across the age, body weight, disease severity, and dose subgroups and was sustained until Week 52. Llimited studies have reported the efficacy of treatments in subgroups of pediatric patients with psoriasis [17, 18]. Two studies have reported comparable findings for ixekizumab and etanercept in pediatric patients with moderate to severe plaque psoriasis aged 6 to < 18 and 4 to < 18 years, respectively [17, 18].
Secukinumab was well tolerated at both dosing regimens, and the overall safety profile was consistent with the safety profile established in adult studies [8, 10, 19]. There were no new or unexpected safety signals. No dose-dependent increases were observed for the incidence of overall AEs, SAEs, AEs leading to study drug discontinuation/interruption, or AEs of interest.
Further long-term data for safety are being collected up to 224 weeks, including a follow-up period of 16 weeks. In this study, the lower age of enrollment was limited to 6 years because the prevalence of psoriasis in the < 6-year-old age group is very low (with the highest prevalence published as 0.3%), and the proportion of pediatric patients with a severe condition in need of systemic treatment is 4.0%, giving a final prevalence of approximately 1 per 10,000 in this age group [20].
The findings of the current study should be interpreted considering the limitations of the open-label design, small sample size, and the lack of a sample group. However, given the limited number of approved treatments available for pediatric patients, compared with adults, the strength of this study is the availability of long-term safety and efficacy data for secukinumab in pediatric patients, including efficacy in subgroups.
5 Conclusion
Both LD and HD of secukinumab demonstrated high and sustained efficacy up to Week 52 and improved health-related QoL in pediatric patients with moderate to severe plaque psoriasis. The safety profile of secukinumab in this study was consistent with that established in studies in adult patients with psoriasis. The results for various subgroups by age, body weight, disease severity, and LD and HD groups demonstrated the high and sustained efficacy of secukinumab in pediatric patients with moderate to severe plaque psoriasis up to Week 52.
References
Burden-Teh E, Thomas KS, Ratib S, Grindlay D, Adaji E, Murphy R. The epidemiology of childhood psoriasis: a scoping review. Br J Dermatol. 2016;174(6):1242–57.
Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155(1):145–51.
Kara T, Topkarci Z, Yilmaz S, Akaltun I, Erdogan B. Pediatric patients with psoriasis and psychiatric disorders: premorbidity and comorbidity in a case-control study. J Dermatolog Treat. 2019;30(2):129–34.
Ustekinumab prescribing information. 2020 [cited 2021 October]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125261s150lbl.pdf. Accessed 5 Oct 2021.
Bronckers I, Paller AS, West DP, Lara-Corrales I, Tollefson MM, Tom WL, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156(4):384–92.
Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration (FDA). The Voice of the Patient. 2016 [cited 2021 05 May]. https://www.fda.gov/media/101758/download. Accessed 5 May 2021.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–9.
Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(1):27 e1-36 e1.
Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, Xia S, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–14.
Novartis Cosentyx® receives EU approval for first-line systemic treatment in pediatric psoriasis. 2020 August 3, 2020 [cited 2021 May 11]. https://www.novartis.com/news/media-releases/novartis-cosentyx-receives-eu-approval-first-line-systemic-treatment-pediatric-psoriasis. Accessed 11 May 2021.
Novartis Cosentyx receives FDA approval for treatment of children and adolescents with moderate to severe plaque psoriasis. 2021 [cited 2021 July 2021]. https://www.novartis.com/news/media-releases/novartis-cosentyx-receives-fda-approval-treatment-children-and-adolescents-moderate-severe-plaque-psoriasis. Accessed 8 July 2021.
Magnolo N, Kingo K, Laquer V, Browning J, Reich A, Szepietowski JC, et al. A phase III open-label, randomized multicenter study to evaluate efficacy and safety of secukinumab in pediatric patients with moderate to severe plaque psoriasis: 24-week results. J Am Acad Dermatol. 2022;86(1):122–30.
Thaçi D, Puig L, Reich K, Tsai TF, Tyring S, Kingo K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: Results from the CLEAR study. J Am Acad Dermatol. 2019;81(6):1405–9.
Bodemer C, Kaszuba A, Kingo K, Tsianakas A, Morita A, Rivas E, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938–47.
Paller AS, Seyger MMB, Alejandro Magariños G, Bagel J, Pinter A, Cather J, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183(2):231–41.
Paller AS, Eichenfield LF, Langley RG, Leonardi CL, Siegfried EC, Creamer K, et al. Subgroup analyses of etanercept in pediatric patients with psoriasis. J Am Acad Dermatol. 2010;63(2):e38-41.
Deodhar A, Mease PJ, McInnes IB, Baraliakos X, Reich K, Blauvelt A, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111.
European Medicines Agency. Assessment Report, Cosentyx. 2020 June 25, 2020 [cited 2021 May 11]. https://www.ema.europa.eu/en/documents/variation-report/cosentyx-h-c-3729-ii-0057-epar-assessment-report-variation_en.pdf. Accessed 11 May 2021.
Acknowledgements
The authors thank Sandeep Subramaniam, Avinash Thakur, and Bitumani Borah (Novartis Health Care Pvt. Ltd., Hyderabad, India) for editorial and medical writing support, which was funded by Novartis Pharma AG, Basel, Switzerland in accordance with the good publication practice guidelines (http://www.ismpp.org/gpp3).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This investigation was sponsored by Novartis Pharma AG, Basel, Switzerland. Nina Magnolo, Külli Kingo, Vivian Laquer, John Browning, Adam Reich, Jacek C. Szepietowski, Deborah Keefe, Philemon Papanastasiou, Weibin Bao, Pascal Forrer, Manmath Patekar
Conflicts of Interest
N. Magnolo has been a principal investigator in studies performed by AbbVie, Almirall, Asana, Boehringer Ingelheim, BMS, Celgene, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Genentech, Incyte, Janssen, Kyowa Kirin, LEO Pharma, Novartis, MSD, Pfizer, Regeneron, Sun Pharma, and UCB and is a consultant or speaker for AbbVie, Almirall, Boehringer Ingelheim, Eli Lilly, LEO Pharma, Novartis, Pfizer, and UCB. K. Kingo has received fees for serving as an investigator in studies sponsored by Celgene, Merck, Mitsubishi Tanabe Pharma, Novartis, Regeneron Pharmaceuticals, and Sandoz. V. Laquer is an investigator for AbbVie, Amgen, Biofrontera, Cara Therapeutics, Celgene, ChemoCentryx, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Kiniksa, LEO Pharma, Novartis, Pfizer, Regeneron, Sun Pharma, and UCB. J. Browning is an investigator for Amryt, Brickell Biotech, Celgene, ChemoCentryx, Eli Lilly, Incyte, Lenus, LEO Pharma, Mayne, Novartis, Pfizer, Regeneron, and Valeant; a consultant for Dermavant and LEO Pharma; and a speaker for Dermira, Regeneron, and Pfizer. JC. Szepietowski is an advisory board member for AbbVie, LEO Pharma, Novartis, Pierre Fabre, Menlo Theraputics, Sienna Biopharmaceuticans, and Trevi; a principal investigator for AbbVie, Novartis, Menlo Therapeutics, Trevi, Janssen, Merck, Regeneron, Amgen, Boehringer Ingelheim, Galapagos, Galderma, InflaRX, Kymab Ltd., Pfizer, UCB, Helm, and Incyte; and a speaker for AbbVie, Novartis, Janssen, Eli Lilly, Sanofi-Genzyme, Sunfarm, and Berlin-Chemie Mennarini. A. Reich is a principal investigator or subinvestigator in clinical trials sponsored by AbbVie, Drug Delivery Solutions Ltd, Galderma, Genentech, Janssen, Kymab Ltd, LEO Pharma, Menlo Therapeutics, MetrioPharm AG, MSD, Novartis, Pfizer, and Trevi Therapeutics and a consultant or speaker for AbbVie, Bioderma, Celgene, Chema-Elektromet, Eli Lilly, Galderma, Janssen, LEO Pharma, Medac, Menlo Therapeutics, Novartis, Pierre Fabre, Sandoz, and Trevi Therapeutics. D. Keefe and W. Bao are employees of Novartis Pharmaceuticals Corporation. P. Papanastasiou, P. Forrer and M. Patekar are employees of Novartis Pharma AG.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available. Novartis is committed to sharing access to patient-level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) and in compliance with all federal, local, or regional requirements. Institutional review board/Ethics Committees of each participating center approved the study protocol.
Consent
A parent or legal guardian provided written informed consent, and the patient provided written assent, at screening before any assessment was performed. If patients reached age of consent (as per local law) during the study, they needed to also sign the corresponding study Informed Consent(s).
Author contributions
All authors were involved in the drafting and critical review of the manuscript, and approved the final version for submission. NM, KK, VL, JB, AR, and JCS contributed to the design of the study and were involved in the acquisition of clinical data and participated as principal investigators in the clinical study from which data are reported in the manuscript. DK, PP, PF, and MP were involved in the conception, design, and clinical conduct of the trial. WB was involved in the analysis of the data in the manuscript. All authors were involved in the interpretation of data in the manuscript. All authors agreed to be accountable for all aspects of the work and attest to the accuracy and integrity of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Magnolo, N., Kingo, K., Laquer, V. et al. Efficacy of Secukinumab Across Subgroups and Overall Safety in Pediatric Patients with Moderate to Severe Plaque Psoriasis: Week 52 Results from a Phase III Randomized Study. Pediatr Drugs 24, 377–387 (2022). https://doi.org/10.1007/s40272-022-00507-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00507-0