当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switchable photocatalysis for the chemodivergent benzylation of 4-cyanopyridines
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2sc02698h Eleni Georgiou 1, 2 , Davide Spinnato 1, 2 , Kang Chen 1 , Paolo Melchiorre 1, 3 , Kilian Muñiz 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2sc02698h Eleni Georgiou 1, 2 , Davide Spinnato 1, 2 , Kang Chen 1 , Paolo Melchiorre 1, 3 , Kilian Muñiz 1, 2, 3
Affiliation
|
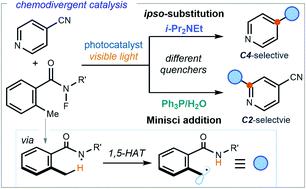
We report a photocatalytic strategy for the chemodivergent radical benzylation of 4-cyanopyridines. The chemistry uses a single photoredox catalyst to generate benzyl radicals upon N–F bond activation of 2-alkyl N-fluorobenzamides. The judicious choice of different photocatalyst quenchers allowed us to select at will between mechanistically divergent processes. The two reaction manifolds, an ipso-substitution path proceeding via radical coupling and a Minisci-type addition, enabled selective access to regioisomeric C4 or C2 benzylated pyridines, respectively. Mechanistic investigations shed light on the origin of the chemoselectivity switch.
中文翻译:

4-氰基吡啶化学发散苄基化的可切换光催化
我们报告了一种用于 4-氰基吡啶的化学发散自由基苄基化的光催化策略。该化学过程使用单一的光氧化还原催化剂在 2-烷基N-氟苯甲酰胺的 N-F 键活化时产生苄基自由基。不同光催化剂猝灭剂的明智选择使我们能够在机械不同的过程之间随意选择。这两个反应歧管,通过自由基偶联和Minisci型加成进行的同位取代路径,分别能够选择性地获得区域异构 C4 或 C2 苄基化吡啶。机理研究揭示了化学选择性开关的起源。
更新日期:2022-06-07
中文翻译:

4-氰基吡啶化学发散苄基化的可切换光催化
我们报告了一种用于 4-氰基吡啶的化学发散自由基苄基化的光催化策略。该化学过程使用单一的光氧化还原催化剂在 2-烷基N-氟苯甲酰胺的 N-F 键活化时产生苄基自由基。不同光催化剂猝灭剂的明智选择使我们能够在机械不同的过程之间随意选择。这两个反应歧管,通过自由基偶联和Minisci型加成进行的同位取代路径,分别能够选择性地获得区域异构 C4 或 C2 苄基化吡啶。机理研究揭示了化学选择性开关的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号