Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2021-02-28 , DOI: 10.2174/1570180817999200802031547 Siphesihle Jama 1 , Xhamla Nqoro 1 , Eric Morifi 2 , Blessing Atim Aderibigbe 1
|
Background: Malaria is a deadly and infectious disease responsible for millions of death worldwide, mostly in the African region. The malaria parasite has developed resistance to the currently used antimalarial drugs, and it has urged researchers to develop new strategies to overcome this challenge by designing different classes of antimalarials.
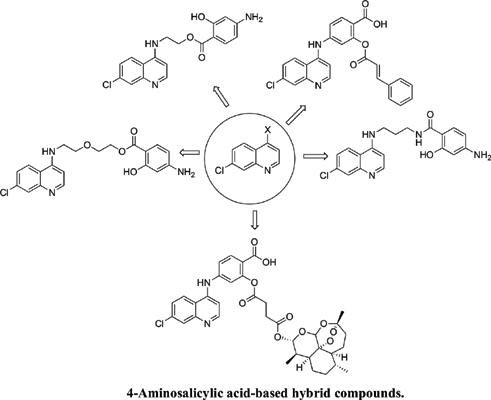
Objectives: A class of hybrid compounds containing 4-aminosalicylic acid moiety was prepared via esterification and amidation reactions and characterized using FTIR, NMR and LC-MS. In vitro antiplasmodial evaluation was performed against the asexual NF54 strain of P. falciparum parasites.
Methods: In this research, known 4-aminoquinoline derivatives were hybridized with 4- aminosalicylic acid to afford hybrid compounds via esterification and amidation reactions. 4- aminosalicylic acid, a dihydrofolate compound inhibits DNA synthesis in the folate pathway and is a potential pharmacophore for the development of antimalarials.
Results: The LC-MS, FTIR, and NMR analysis confirmed the successful synthesis of the compounds. The compounds were obtained in yields in the range of 63-80%. The hybrid compounds displayed significant antimalarial activity when compared to 4-aminosalicylic acid, which exhibited poor antimalarial activity. The IC50 value of the most potent hybrid compound, 9 was 9.54±0.57 nm.
Conclusion: 4-aminosalicylic has different functionalities, which can be used for hybridization with a wide range of compounds. It is a potential pharmacophore that can be utilized for the design of potent antimalarial drugs. It was found to be a good potentiating agent when hybridized with 4- aminoquinoline derivatives suggesting that they can be utilized for the synthesis of a new class of antimalarials.
中文翻译:

基于4-氨基水杨酸的杂化化合物:合成和体外抗血浆评价
背景:疟疾是一种致命的传染性疾病,在世界范围内(主要在非洲地区)造成数百万人死亡。疟原虫对目前使用的抗疟药产生了抗药性,并敦促研究人员通过设计不同类别的抗疟药来开发新的策略来克服这一挑战。
目的:通过酯化和酰胺化反应制备了一类含有4-氨基水杨酸部分的杂化化合物,并利用FTIR,NMR和LC-MS对其进行了表征。针对恶性疟原虫寄生虫的无性NF54菌株进行了体外抗疟原虫评估。
方法:在这项研究中,将已知的4-氨基喹啉衍生物与4-氨基水杨酸杂交,通过酯化和酰胺化反应得到杂化化合物。4-氨基水杨酸,一种二氢叶酸化合物,可抑制叶酸途径中的DNA合成,是开发抗疟疾药的潜在药效团。
结果:LC-MS,FTIR和NMR分析证实了化合物的成功合成。获得的化合物的产率为63-80%。与4-氨基水杨酸相比,杂合化合物显示出显着的抗疟活性,后者显示出较差的抗疟活性。最有效的杂化化合物9的IC50值为9.54±0.57 nm。
结论:4-氨基水杨酸具有不同的功能,可用于与多种化合物的杂交。它是一种潜在的药效基团,可用于设计有效的抗疟药。当与4-氨基喹啉衍生物杂交时,发现它是一种良好的增强剂,表明它们可用于合成一类新的抗疟疾药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号