Frontiers of Chemical Science and Engineering ( IF 4.3 ) Pub Date : 2021-04-07 , DOI: 10.1007/s11705-020-2034-6 Chuangnian Zhang , Ying Dong , Jing Gao , Xiaoli Wang , Yanjun Jiang
|
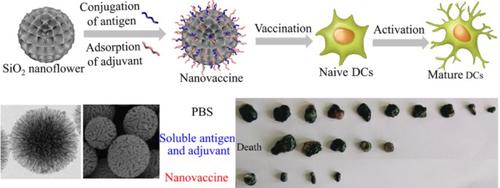
Here, we reported a cancer nanovaccine based on SiO2 nanoflowers with a special radial pore structure, which greatly enhanced cross-presentation and induced the production of cytotoxic T lymphocyte cells secreting granzymes B and interferon-γ. The antigen ovalbumin was covalently conjugated onto the as-synthesized hierarchical SiO2 nanoflowers, and the adjuvant cytosine-phosphate-guanine was electrostatically adsorbed into their radial pore by simple mixing before use. The nanovaccine exhibited excellent storage stability without antigen release after 27 days of incubation, negligible cytotoxicity to dendritic cells, and a high antigen loading capacity of 430 ± 66 mg·g−1 support. Besides, the nanovaccine could be internalized by dendritic cells via multiple pathways. And the enhancement of antigen/adjuvant uptake and lysosome escape of antigen were observed. Noteworthy, in vitro culture of bone marrow-derived dendritic cells in the presence of nanovaccine proved the activation of dendritic cells and antigen cross-presentation as well as secretion of proinflammatory cytokines. Besides, in vivo study verified the targeting of nanovaccine to draining lymph nodes, the complete suppression of tumor in six out of ten mice, and the triggering of notable tumor growth delay. Overall, the present results indicated that the nanovaccine can be served as a potential therapeutic vaccine to treat cancer.
中文翻译:

径向多孔SiO 2纳米花增强抗原/佐剂在抗肿瘤免疫治疗中的作用
在这里,我们报道了一种基于具有特殊径向孔结构的SiO 2纳米花的癌症纳米疫苗,该疫苗大大增强了交叉呈递并诱导了分泌颗粒酶B和干扰素γ的细胞毒性T淋巴细胞的产生。将抗原卵清蛋白共价结合到合成后的分级SiO 2纳米花上,并在使用前通过简单混合将辅助的胞嘧啶-磷酸-鸟嘌呤静电吸附到其径向孔中。温育27天后,纳米疫苗显示出优异的储存稳定性而无抗原释放,对树突状细胞的细胞毒性可忽略不计,并且具有430±66 mg·g -1的高抗原载量支持。此外,纳米疫苗可以通过多种途径被树突状细胞内化。并观察到抗原/佐剂摄取的增强和抗原的溶酶体逸出。值得注意的是,在纳米疫苗存在下骨髓来源的树突状细胞的体外培养证明了树突状细胞的激活和抗原交叉呈递以及促炎性细胞因子的分泌。此外,体内研究证实了纳米疫苗靶向引流淋巴结,十只小鼠中的六只完全抑制了肿瘤,并触发了显着的肿瘤生长延迟。总体而言,目前的结果表明,纳米疫苗可以作为潜在的治疗性疫苗来治疗癌症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号