Journal of Advanced Research ( IF 11.4 ) Pub Date : 2021-03-31 , DOI: 10.1016/j.jare.2021.03.012 Chang Qu 1 , Qiao-Ping Li 2 , Zi-Ren Su 2 , Siu-Po Ip 1, 3 , Qiu-Ju Yuan 1, 3 , You-Liang Xie 2 , Qing-Qing Xu 1 , Wen Yang 1 , Yan-Feng Huang 1 , Yan-Fang Xian 1, 3 , Zhi-Xiu Lin 1, 3, 4
|
Introduction
Honokiol (HO) exerts neuroprotective effects in several animal models of Alzheimer’s disease (AD), but the poor dissolution hampers its bioavailability and therapeutic efficacy.
Objectives
A novel honokiol nanoscale drug delivery system (Nano-HO) with smaller size and excellent stability was developed in this study to improve the solubility and bioavailability of HO. The anti-AD effects of Nano-HO was determined.
Methods
Male TgCRND8 mice were daily orally administered Nano-HO or HO at the same dosage (20 mg/kg) for 17 consecutive weeks, followed by assessment of the spatial learning and memory functions using the Morris Water Maze test (MWMT).
Results
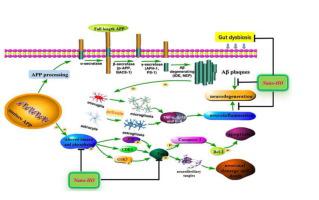
Our pharmacokinetic study indicated that the oral bioavailability was greatly improved by Nano-HO. In addition, Nano-HO significantly improved cognitive deficits and inhibited neuroinflammation via suppressing the levels of TNF-α, IL-6 and IL-1β in the brain, preventing the activation of microglia (IBA-1) and astrocyte (GFAP), and reducing β-amyloid (Aβ) deposition in the cortex and hippocampus of TgCRND8 mice. Moreover, Nano-HO was more effective than HO in modulating amyloid precursor protein (APP) processing via suppressing β-secretase, as well as enhancing Aβ-degrading enzymes like neprilysin (NEP). Furthermore, Nano-HO more markedly inhibited tau hyperphosphorylation via decreasing the ratio of p-Tau (Thr 205)/tau and regulating tau-related apoptosis proteins (caspase-3 and Bcl-2). In addition, Nano-HO more markedly attenuated the ratios of p-JNK/JNK and p-35/CDK5, while enhancing the ratio of p-GSK-3β (Ser9)/GSK-3β. Finally, Nano-HO prevented the gut microflora dysbiosis in TgCRND8 mice in a more potent manner than free HO.
Conclusion
Nano-HO was more potent than free HO in improving cognitive impairments in TgCRND8 mice via inhibiting Aβ deposition, tau hyperphosphorylation and neuroinflammation through suppressing the activation of JNK/CDK5/GSK-3β signaling pathway. Nano-HO also more potently modulated the gut microbiota community to protect its stability than free HO. These results suggest that Nano-HO has good potential for further development into therapeutic agent for AD treatment.
中文翻译:

Nano-Honokiol 通过抑制神经病理学和调节肠道菌群改善阿尔茨海默病 TgCRND8 小鼠的认知缺陷
介绍
和厚朴酚 (H2O) 在阿尔茨海默病 (AD) 的几种动物模型中发挥神经保护作用,但溶解度差阻碍了其生物利用度和治疗效果。
目标
本研究开发了一种新型的和厚朴酚纳米级药物递送系统(Nano-H2O),其尺寸更小、稳定性好,以提高 H2O 的溶解度和生物利用度。测定了 Nano-H2O 的抗 AD 作用。
方法
雄性 TgCRND8 小鼠每天口服相同剂量(20 mg/kg)的 Nano-H2O 或 H2O,连续 17 周,然后使用莫里斯水迷宫测试(MWMT)评估空间学习和记忆功能。
结果
我们的药代动力学研究表明,纳米 H2O 大大提高了口服生物利用度。此外,Nano-HO 通过抑制大脑中 TNF-α、IL-6 和 IL-1β 的水平,防止小胶质细胞 (IBA-1) 和星形胶质细胞 (GFAP) 的激活,显着改善认知缺陷并抑制神经炎症,以及减少 TgCRND8 小鼠皮质和海马中的 β-淀粉样蛋白 (Aβ) 沉积。此外,纳米 H2O 比 H2O 更有效地通过抑制 β-分泌酶来调节淀粉样前体蛋白 (APP) 的加工,以及增强像中性溶酶 (NEP) 这样的 Aβ 降解酶。此外,Nano-H2O 通过降低 p-Tau (Thr 205)/tau 的比率和调节 tau 相关的凋亡蛋白(caspase-3 和 Bcl-2)更显着地抑制 tau 过度磷酸化。此外,Nano-H2O 更显着地减弱了 p-JNK/JNK 和 p-35/CDK5 的比例,同时提高了 p-GSK-3β (Ser9)/GSK-3β 的比例。最后,Nano-H2O 以比游离 H2O 更有效的方式预防 TgCRND8 小鼠肠道菌群失调。
结论
Nano-H2O 比游离 H2O 更有效地通过抑制 JNK/CDK5/GSK-3β 信号通路的激活来抑制 Aβ 沉积、tau 过度磷酸化和神经炎症来改善 TgCRND8 小鼠的认知障碍。Nano-H2O 还比游离 H2O 更有效地调节肠道微生物群落以保护其稳定性。这些结果表明,Nano-H2O 具有良好的潜力,可进一步发展成为治疗 AD 的治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号