Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-01-04 , DOI: 10.1007/s10593-020-02847-6 Yavuz Derin , Barış Seçkin Arslan , Büşra Albayrak Mısır , İlkay Şişman , Mehmet Nebioğlu , Ahmet Tutar
|
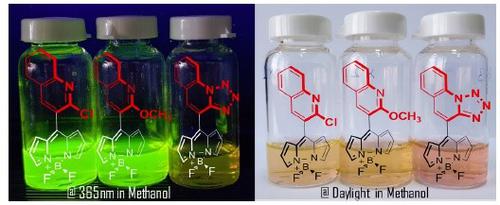
Quinoline-based BODIPY AIEgen dyes were synthesized and the structures were elucidated by 1H, 13C, 19F NMR, FT-IR spectroscopy and mass spectrometry methods. Their photophysical properties were investigated. The dyes showed fluorescence quantum yield in the range of 0.16–1.29% in MeOH. It was found that the presence of methoxy group and tetrazole moiety led to blue and red spectral shift, respectively, of the UV absorption maxima of these dyes compared to their chloroquinoline analog. Stokes shifts of the dyes were in the range of 637–955 cm–1. Aggregation-induced emission behavior of the dyes was investigated in EtOH–H2O mixture so that the dyes exhibited 1.6- to 2.3-fold fluorescence enhancement.
中文翻译:

载有喹啉和BODIPY支架的AIEgen染料的合成和光物理研究
合成了基于喹啉的BODIPY AIEgen染料,并通过1 H,13 C,19 F NMR,FT-IR光谱和质谱法阐明了结构。研究了它们的光物理性质。染料在MeOH中的荧光量子产率为0.16-1.29%。已经发现,与它们的氯喹啉类似物相比,甲氧基和四唑部分的存在分别导致这些染料的最大紫外吸收光谱的蓝和红光谱偏移。染料的斯托克斯位移范围为637–955 cm –1。在EtOH–H 2 O混合物中研究了染料的聚集诱导的发射行为,从而使染料表现出1.6至2.3倍的荧光增强。











































 京公网安备 11010802027423号
京公网安备 11010802027423号