当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In vitro and in vivo osteogenesis up-regulated by two-dimensional nanosheets through a macrophage-mediated pathway
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-11-9 , DOI: 10.1039/d0bm01596b Haoming Liu 1, 2, 3, 4, 5 , Gaojie Yang 5, 6, 7, 8 , Hao Yin 1, 2, 3, 4, 5 , Zhenxing Wang 1, 2, 3, 4, 5 , Chunyuan Chen 1, 2, 3, 4, 5 , Zhengzhao Liu 2, 3, 4, 5, 9 , Hui Xie 1, 2, 3, 4, 5
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-11-9 , DOI: 10.1039/d0bm01596b Haoming Liu 1, 2, 3, 4, 5 , Gaojie Yang 5, 6, 7, 8 , Hao Yin 1, 2, 3, 4, 5 , Zhenxing Wang 1, 2, 3, 4, 5 , Chunyuan Chen 1, 2, 3, 4, 5 , Zhengzhao Liu 2, 3, 4, 5, 9 , Hui Xie 1, 2, 3, 4, 5
Affiliation
|
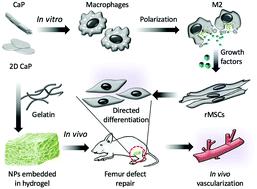
Two-dimensional (2D) nanomaterials are attracting more and more interest in regenerative medicine due to their unique properties; however 2D biomimetic calcium mineral has not yet been developed and demonstrated application for bone tissue engineering. Here we described a novel calcium phosphate material with a 2D nanostructure that was synthesized using collagen and sodium alginate as the template. In vitro performance of the nanocrystalline material was evaluated, and we found that 2D CaP nanoparticles (NPs) enhanced the in vitro osteogenic differentiation of rat mesenchymal stem cells (rMSCs) through a macrophage-mediated signal pathway, when co-cultured with RAW 264.7 cells, rather than direct NP/stem cell interaction. A 2D topology structured surface was constructed by encapsulating the CaP nanomaterials in a gelatin hydrogel, which was demonstrated to be able to mediate in vivo ossification through a macrophage polarization related pathway in a femur defect rat model, and allowed the optimal therapeutic outcome compared to normal CaP counterparts. Our current work may have enlightened a new mechanism regarding NP-induced stem cell differentiation through immunoregulation, and the 2D CaP encapsulated hydrogel scaffold may serve as a potential alternative to autograft bone for orthopedic applications.
中文翻译:

二维和纳米片通过巨噬细胞介导的途径上调体外和体内成骨作用
二维(2D)纳米材料由于其独特的性能而引起了越来越多的对再生医学的兴趣。然而,尚未开发出2D仿生钙矿物质并证明了其在骨组织工程中的应用。在这里,我们描述了一种具有2D纳米结构的新型磷酸钙材料,该材料使用胶原蛋白和藻酸钠作为模板合成。评价了纳米晶体材料的体外性能,我们发现二维CaP纳米颗粒(NPs)增强了体外与RAW 264.7细胞共培养时,通过巨噬细胞介导的信号途径使大鼠间充质干细胞(rMSCs)成骨分化,而不是直接NP /干细胞相互作用。通过将CaP纳米材料封装在明胶水凝胶中来构建2D拓扑结构化表面,该表面被证明能够在股骨缺损大鼠模型中通过巨噬细胞极化相关途径介导体内骨化,并且与正常情况相比具有最佳治疗效果CaP同行。我们当前的工作可能已经启发了有关通过免疫调节来诱导NP诱导干细胞分化的新机制,而2D CaP封装的水凝胶支架可能会成为自体骨在骨科应用中的潜在替代物。
更新日期:2020-12-16
中文翻译:

二维和纳米片通过巨噬细胞介导的途径上调体外和体内成骨作用
二维(2D)纳米材料由于其独特的性能而引起了越来越多的对再生医学的兴趣。然而,尚未开发出2D仿生钙矿物质并证明了其在骨组织工程中的应用。在这里,我们描述了一种具有2D纳米结构的新型磷酸钙材料,该材料使用胶原蛋白和藻酸钠作为模板合成。评价了纳米晶体材料的体外性能,我们发现二维CaP纳米颗粒(NPs)增强了体外与RAW 264.7细胞共培养时,通过巨噬细胞介导的信号途径使大鼠间充质干细胞(rMSCs)成骨分化,而不是直接NP /干细胞相互作用。通过将CaP纳米材料封装在明胶水凝胶中来构建2D拓扑结构化表面,该表面被证明能够在股骨缺损大鼠模型中通过巨噬细胞极化相关途径介导体内骨化,并且与正常情况相比具有最佳治疗效果CaP同行。我们当前的工作可能已经启发了有关通过免疫调节来诱导NP诱导干细胞分化的新机制,而2D CaP封装的水凝胶支架可能会成为自体骨在骨科应用中的潜在替代物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号