当前位置:
X-MOL 学术
›
Curr. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent Developments in the Asymmetric Detrifluoroacetylative Reactions of in situ Generated Mono-Fluorinated Enolates
Current Organic Chemistry ( IF 1.7 ) Pub Date : 2020-08-31 , DOI: 10.2174/1385272824999200801022712 Li Wang 1 , Ziyi Li 1 , Jiang Liu 1 , Jianlin Han 1 , Hiroki Moriwaki 2 , Vadim A. Soloshonok 3
中文翻译:

原位生成的单氟烯醇酸酯不对称三氟乙酰化反应的最新进展
更新日期:2020-08-31
Current Organic Chemistry ( IF 1.7 ) Pub Date : 2020-08-31 , DOI: 10.2174/1385272824999200801022712 Li Wang 1 , Ziyi Li 1 , Jiang Liu 1 , Jianlin Han 1 , Hiroki Moriwaki 2 , Vadim A. Soloshonok 3
Affiliation
|
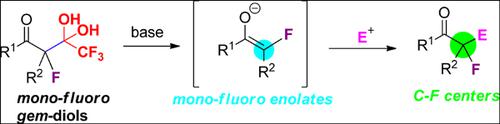
The development of an efficient and mild synthetic methodology for the construction of bioactive fluorine-containing molecules represents one of the hot research topics in general synthetic organic chemistry. In this review, some recent progresses achieved in the development of detrifluoroacetylatively generated mono-fluorinated enolates via CC bond cleavage and their asymmetric nucleophilic reactions for assembly of chiral quaternary C-F center containing compounds.
中文翻译:

原位生成的单氟烯醇酸酯不对称三氟乙酰化反应的最新进展
用于构建生物活性含氟分子的高效温和的合成方法的开发代表了一般合成有机化学中的热门研究主题之一。在这篇综述中,通过CC键裂解及其在用于手性季CF中心的化合物的组装中的不对称亲核反应,开发了由三氟乙酰化生成的单氟化烯醇酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号