Medicinal Chemistry ( IF 2.3 ) Pub Date : 2021-06-30 , DOI: 10.2174/1573406416666191227115451 Min Gao 1 , QiaoLi Lv 2 , HouPan Zhang 1 , GuoGang Tu 1
|
Background: As a target for anticancer treatment, aminopeptidase N (APN) shows its overexpression on diverse malignant tumor cells and associates with cancer invasion, angiogenesis and metastasis.
Objective: The objective of the study was the design, synthesis and biological activity evaluation of alanine hydroxamic acid derivatives as APN inhibitors, and investigation of the binding mode of inhibitors in the APN active site.
Methods: Alanine hydroxamic acid derivatives were synthesized and evaluated for their in vitro anti-cancer activity using CCK-8 assay. Molecular docking and 4D-QSAR studies were carried out to suggest the mechanism of biological activity.
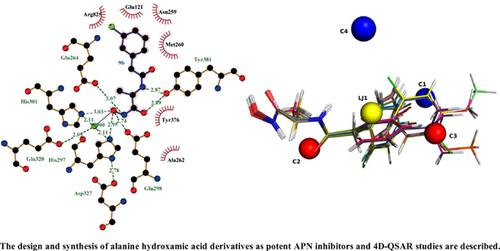
Results: Compared with Bestatin, compound 9b showed the best APN inhibition activity. The putative binding mode of 9b in the APN active site was also discussed. Moreover, the robust and reliable 4D-QSAR model exhibited the following statistics: R2 = 0.9352, q2 LOO = 0.8484, q2 LNO =0.7920, R2 Pred = 0.8739.
Conclusion: Newly synthesized compounds exerted acceptable anticancer activity and further investigation of the current scaffold would be beneficial.
中文翻译:

作为氨肽酶 N 抑制剂的丙氨酸异羟肟酸衍生物的合成和 4D-QSAR 研究
背景:作为抗癌治疗的靶点,氨肽酶 N (APN) 在多种恶性肿瘤细胞中过度表达,并与癌症侵袭、血管生成和转移有关。
目的:本研究的目的是设计、合成作为 APN 抑制剂的丙氨酸异羟肟酸衍生物的设计、合成和生物活性评价,并研究抑制剂在 APN 活性位点的结合模式。
方法:合成丙氨酸异羟肟酸衍生物,并使用 CCK-8 测定评估其体外抗癌活性。进行了分子对接和 4D-QSAR 研究以提出生物活性的机制。
结果:与Bestatin相比,化合物9b表现出最好的APN抑制活性。还讨论了 APN 活性位点中 9b 的假定结合模式。此外,稳健可靠的 4D-QSAR 模型表现出以下统计数据:R 2 = 0.9352,q 2 LOO = 0.8484,q 2 LNO =0.7920,R 2 Pred = 0.8739。
结论:新合成的化合物具有可接受的抗癌活性,对当前支架的进一步研究将是有益的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号