Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-07-31 , DOI: 10.2174/1573406416666200420080459 Zeynep Ates-Alagoz 1 , Mehmet Murat Kisla 1 , Hakan Goker 1 , Sulhiye Yildiz 2
|
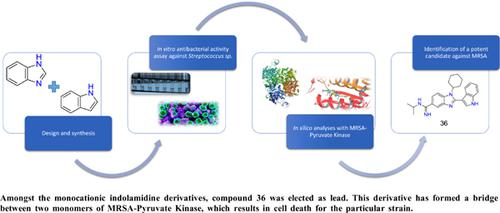
Background: Finding efficient therapy against hospital-acquired MRSA infections has become rather important in the last decade. To this end, inhibition of the enzyme pyruvate kinase (PK) is being investigated for antibacterial activity, since this enzyme controls energy generation and metabolic flux distribution. Our main scaffold consists of benzimidazole and indole rings fused together. Both rings are famous for antibacterial properties and promising anti-MRSA compounds including indole ring.
Methods: Several 1-substituted-2-(1H-indol-3-yl)-N-substituted-1H-benzimidazole-5-carboxamidine analogues were developed, synthesized and their antibacterial activities were evaluated against Staphylococcus aureus (ATCC 25923), Methicillin resistant Staphylococcus aureus (MRSA) (ATCC 43300), and Staphylococcus epidermidis (ATCC 12228) by using tube dilution method. Molecular docking analysis with a characteristic protein called MRSA- Pyruvate Kinase has been conducted for the assessment of the activities of our compounds against Methicillinresistant S. aureus (MRSA).
Results: Among all the tested compounds, the most potent compound 36 had MIC values as 3.12, 3.12 and 6.25 μg/mL against S. aureus, Methicillin-resistant S. aureus (MRSA), and S. epidermidis, respectively. This compound had much better docking energy value than standard ampicillin and also created the link between two residues in different monomers of PK.
Discussion: This approach of using indol-amidine conjugate systems as anti-MRSA agents may include MRSA-PK as potential target. To further increase the affinity, some other H-bonding parts may be added. By doing so, another bridge with Ile361 residues on both sides can be created. Our compounds tend to violate log P limit of Lipinski, therefore some optimizations with formulation can be made.
Conclusion: This study mainly includes the design, synthesis and optimization of indolebenzimidazole- amidine derivatives. Docking studies confirmed our results, since our most potent hit compound 36 created the necessary interactions between two chains of MRSA-PK. Further optimization can be considered to increase drug ability.
中文翻译:

新型单阳离子吲哚-苯并咪唑衍生物的合成、分子对接研究及抗菌活性
背景:在过去十年中,寻找针对医院获得性 MRSA 感染的有效疗法变得相当重要。为此,正在研究抑制丙酮酸激酶 (PK) 的抗菌活性,因为该酶控制能量产生和代谢通量分布。我们的主要支架由融合在一起的苯并咪唑和吲哚环组成。这两个环都以抗菌特性和有前途的抗 MRSA 化合物(包括吲哚环)而闻名。
方法:开发、合成了几种 1-取代-2-(1H-吲哚-3-基)-N-取代-1H-苯并咪唑-5-甲脒类似物,并评估了它们对金黄色葡萄球菌 (ATCC 25923)、甲氧西林的抗菌活性使用试管稀释法对金黄色葡萄球菌 (MRSA) (ATCC 43300) 和表皮葡萄球菌 (ATCC 12228) 产生耐药性。使用称为 MRSA-丙酮酸激酶的特征蛋白进行了分子对接分析,以评估我们的化合物对耐甲氧西林金黄色葡萄球菌 (MRSA) 的活性。
结果:在所有测试化合物中,最有效的化合物 36 对金黄色葡萄球菌、耐甲氧西林金黄色葡萄球菌 (MRSA) 和表皮葡萄球菌的 MIC 值分别为 3.12、3.12 和 6.25 μg/mL。该化合物比标准氨苄青霉素具有更好的对接能量值,并且还在 PK 的不同单体中的两个残基之间建立了联系。
讨论:这种使用吲哚-脒缀合物系统作为抗 MRSA 药物的方法可能包括 MRSA-PK 作为潜在目标。为了进一步增加亲和力,可以添加一些其他的 H 键合部分。通过这样做,可以创建另一个两侧带有 Ile361 残基的桥。我们的化合物往往会违反 Lipinski 的 log P 限制,因此可以对配方进行一些优化。
结论:本研究主要包括吲哚苯并咪唑脒衍生物的设计、合成和优化。对接研究证实了我们的结果,因为我们最有效的命中化合物 36 在 MRSA-PK 的两条链之间产生了必要的相互作用。可以考虑进一步优化以增加药物能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号