当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium(II)-catalyzed acyloxylation of the ortho-C–H bond in 2-aroyl-imidazoles with carboxylic acids
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-31 , DOI: 10.1039/d0qo00920b Chen-an Wang 1, 2, 3, 4, 5 , Naoto Chatani 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-31 , DOI: 10.1039/d0qo00920b Chen-an Wang 1, 2, 3, 4, 5 , Naoto Chatani 1, 2, 3, 4, 5
Affiliation
|
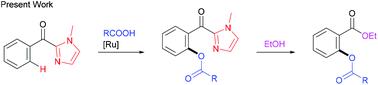
The reaction of 2-aroyl-imidazoles with carboxylic acids using [RuCl2(p-cymene)]2 as the catalyst and Ag2CO3 as the oxidant results in ortho-C–H acyloxylation to afford acyloxylation products, in which the imidazole group functions as a directing group. A wide range of functional groups are tolerated in the reaction. The directing group can be easily converted to the corresponding esters under mild conditions.
中文翻译:

钌(II)催化2-芳酰基咪唑中邻位C–H键与羧酸的酰氧基化
以[RuCl 2(p- Cymene)] 2为催化剂,Ag 2 CO 3为氧化剂,使2-芳酰基-咪唑与羧酸反应,导致邻-C-H酰氧基化,得到酰氧基化产物,其中咪唑组充当指挥组。反应中可以容忍多种官能团。在温和条件下,该导向基团可以容易地转化成相应的酯。
更新日期:2020-09-30
中文翻译:

钌(II)催化2-芳酰基咪唑中邻位C–H键与羧酸的酰氧基化
以[RuCl 2(p- Cymene)] 2为催化剂,Ag 2 CO 3为氧化剂,使2-芳酰基-咪唑与羧酸反应,导致邻-C-H酰氧基化,得到酰氧基化产物,其中咪唑组充当指挥组。反应中可以容忍多种官能团。在温和条件下,该导向基团可以容易地转化成相应的酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号