当前位置:
X-MOL 学术
›
Nat. Protoc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobaltaelectro-catalyzed C-H activation for resource-economical molecular syntheses.
Nature Protocols ( IF 13.1 ) Pub Date : 2020-04-15 , DOI: 10.1038/s41596-020-0306-8 Cong Tian 1 , Tjark H Meyer 1 , Maximilian Stangier 1 , Uttam Dhawa 1 , Karsten Rauch 1 , Lars H Finger 1 , Lutz Ackermann 1
Nature Protocols ( IF 13.1 ) Pub Date : 2020-04-15 , DOI: 10.1038/s41596-020-0306-8 Cong Tian 1 , Tjark H Meyer 1 , Maximilian Stangier 1 , Uttam Dhawa 1 , Karsten Rauch 1 , Lars H Finger 1 , Lutz Ackermann 1
Affiliation
|
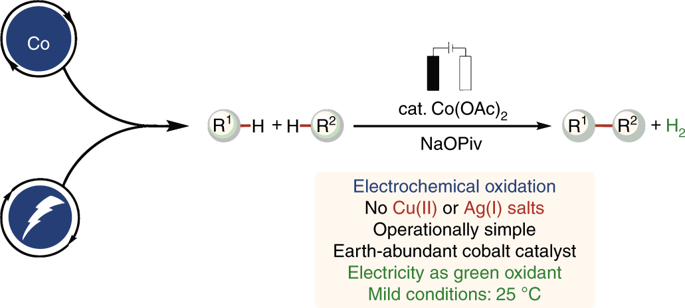
The direct cleavage of otherwise inert C-H bonds has emerged as a sustainable approach for organic synthesis; in contrast to other approaches, these reactions result in the formation of fewer undesired by-products and do not require pre-functionalization steps. In recent years, oxidative C-H/N-H alkyne annulations and C-H oxygenations were realized by 3d metals. Unfortunately, most of these reactions require stoichiometric amounts of often toxic chemical oxidants. This protocol provides a general method for cobaltaelectro-catalyzed C-H activations of benzamides. Here, anodic oxidation obviates the need for a chemical oxidant and uses 10-20% of a more environmentally benign, inexpensive catalyst. We outline a detailed and precise description of the designed electrolytic cell for metallaelectrocatalysis, including readily available electrode materials and electrode holders. The custom-made device is further compared with the commercially available and standardized ElectraSyn 2.0 electrochemistry kit. As example applications of this approach, we describe cobaltaelectro-catalyzed C-H activation protocols for the direct C-H oxygenation of benzamides and resource-economical synthesis of isoquinolones. The cobaltaelectrocatalysis setup and reaction take about 17 h, while an additional 5 h have to be anticipated for workup and chromatographic purification. The methods described herein feature broad functional group tolerance, operational simplicity, low waste-product formation and an overall exceptional level of resource economy.
中文翻译:

钴电子催化的CH活化用于资源经济的分子合成。
直接裂解否则为惰性的CH键已成为有机合成的可持续方法。与其他方法相比,这些反应导致形成更少的不希望的副产物,并且不需要预官能化步骤。近年来,通过3d金属实现了CH / NH炔烃的氧化环氧化和CH氧化。不幸的是,大多数这些反应需要化学计量的经常有毒的化学氧化剂。该方案为钴酰胺催化苯甲酰胺的CH活化提供了一种通用方法。在此,阳极氧化消除了对化学氧化剂的需求,并使用了10-20%的对环境无害且廉价的催化剂。我们概述了所设计的用于金属电催化的电解池的详细而精确的描述,包括现成的电极材料和电极支架。将该定制设备与市售的标准化ElectraSyn 2.0电化学试剂盒进行了进一步比较。作为此方法的示例应用程序,我们描述了用于苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。将该定制设备与市售的标准化ElectraSyn 2.0电化学试剂盒进行了进一步比较。作为此方法的示例应用程序,我们描述了用于苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。将该定制设备与市售的标准化ElectraSyn 2.0电化学试剂盒进行了进一步比较。作为此方法的示例应用程序,我们描述了用于苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。我们描述了苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。我们描述了苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。
更新日期:2020-04-24
中文翻译:

钴电子催化的CH活化用于资源经济的分子合成。
直接裂解否则为惰性的CH键已成为有机合成的可持续方法。与其他方法相比,这些反应导致形成更少的不希望的副产物,并且不需要预官能化步骤。近年来,通过3d金属实现了CH / NH炔烃的氧化环氧化和CH氧化。不幸的是,大多数这些反应需要化学计量的经常有毒的化学氧化剂。该方案为钴酰胺催化苯甲酰胺的CH活化提供了一种通用方法。在此,阳极氧化消除了对化学氧化剂的需求,并使用了10-20%的对环境无害且廉价的催化剂。我们概述了所设计的用于金属电催化的电解池的详细而精确的描述,包括现成的电极材料和电极支架。将该定制设备与市售的标准化ElectraSyn 2.0电化学试剂盒进行了进一步比较。作为此方法的示例应用程序,我们描述了用于苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。将该定制设备与市售的标准化ElectraSyn 2.0电化学试剂盒进行了进一步比较。作为此方法的示例应用程序,我们描述了用于苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。将该定制设备与市售的标准化ElectraSyn 2.0电化学试剂盒进行了进一步比较。作为此方法的示例应用程序,我们描述了用于苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。我们描述了苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。我们描述了苯甲酰胺的直接CH氧化和异喹诺酮类资源经济合成的钴电催化CH活化方案。钴电催化的建立和反应大约需要17小时,而后处理和色谱纯化则需要另外5小时。本文所述的方法的特征在于宽泛的官能团耐受性,操作简便性,低废物形成率以及资源经济的总体优异水平。









































 京公网安备 11010802027423号
京公网安备 11010802027423号