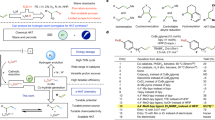
Abstract
The direct cleavage of otherwise inert C–H bonds has emerged as a sustainable approach for organic synthesis; in contrast to other approaches, these reactions result in the formation of fewer undesired by-products and do not require pre-functionalization steps. In recent years, oxidative C–H/N–H alkyne annulations and C–H oxygenations were realized by 3d metals. Unfortunately, most of these reactions require stoichiometric amounts of often toxic chemical oxidants. This protocol provides a general method for cobaltaelectro-catalyzed C–H activations of benzamides. Here, anodic oxidation obviates the need for a chemical oxidant and uses 10–20% of a more environmentally benign, inexpensive catalyst. We outline a detailed and precise description of the designed electrolytic cell for metallaelectrocatalysis, including readily available electrode materials and electrode holders. The custom-made device is further compared with the commercially available and standardized ElectraSyn 2.0 electrochemistry kit. As example applications of this approach, we describe cobaltaelectro-catalyzed C–H activation protocols for the direct C–H oxygenation of benzamides and resource-economical synthesis of isoquinolones. The cobaltaelectrocatalysis setup and reaction take about 17 h, while an additional 5 h have to be anticipated for workup and chromatographic purification. The methods described herein feature broad functional group tolerance, operational simplicity, low waste-product formation and an overall exceptional level of resource economy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and previously published reports (https://doi.org/10.1021/jacs.7b11025 and https://doi.org/10.1002/anie.201712647). Additional data are available from the corresponding author on request.
References
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Gandeepan, P. & Ackermann, L. Transient directing groups for transformative C–H activation by synergistic metal catalysis. Chem 4, 199–222 (2018).
Dey, A., Sinha, S. K., Achar, T. K. & Maiti, D. Accessing remote meta- and para-C(sp2)–H bonds with covalently attached directing groups. Angew. Chem. Int. Ed. 58, 10820–10843 (2019).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Ma, W., Gandeepan, P., Li, J. & Ackermann, L. Recent advances in positional-selective alkenylations: removable guidance for twofold C–H activation. Org. Chem. Front. 4, 1435–1467 (2017).
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
Wencel-Delord, J. & Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010).
Wang, W., Lorion, M. M., Shah, J., Kapdi, A. R. & Ackermann, L. Late-stage peptide diversification by position-selective C–H activation. Angew. Chem. Int. Ed. 57, 14700–14717 (2018).
Noisier, A. F. M. & Brimble, M. A. C–H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 114, 8775–8806 (2014).
Pouliot, J.-R., Grenier, F., Blaskovits, J. T., Beaupré, S. & Leclerc, M. Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem. Rev. 116, 14225–14274 (2016).
Schipper, D. J. & Fagnou, K. Direct arylation as a synthetic tool for the synthesis of thiophene-based organic electronic materials. Chem. Mater. 23, 1594–1600 (2011).
Seki, M. A new catalytic system for Ru-catalyzed C–H arylation reactions and its application in the practical syntheses of pharmaceutical agents. Org. Process Res. Dev. 20, 867–877 (2016).
Ackermann, L. Robust ruthenium(II)-catalyzed C–H arylations: carboxylate assistance for the efficient synthesis of angiotensin-II-receptor blockers. Org. Process Res. Dev. 19, 260–269 (2015).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Meyer, T. H., Finger, L. H., Gandeepan, P. & Ackermann, L. Resource economy by metallaelectrocatalysis: merging electrochemistry and C–H activation. Trends Chem. 1, 63–76 (2019).
Dwivedi, V., Kalsi, D. & Sundararaju, B. Electrochemical-/photoredox aspects of transition metal-catalyzed directed C–H bond activation. ChemCatChem 11, 5160–5187 (2019).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemistry: calling all engineers. Angew. Chem. Int. Ed. 57, 4149–4155 (2018).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Tang, S., Liu, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem 4, 27–45 (2018).
Sauermann, N., Meyer, T. H., Qiu, Y. & Ackermann, L. Electrocatalytic C–H activation. ACS Catal. 8, 7086–7103 (2018).
Sauermann, N., Meyer, T. H. & Ackermann, L. Electrochemical cobalt-catalyzed C–H activation. Chem. Eur. J. 24, 16209–16217 (2018).
Moeller, K. D. Using physical organic chemistry to shape the course of electrochemical reactions. Chem. Rev. 118, 4817–4833 (2018).
Kärkäs, M. D. Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev. 47, 5786–5865 (2018).
Nutting, J. E., Rafiee, M. & Stahl, S. S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 118, 4834–4885 (2018).
Ma, C., Fang, P. & Mei, T.-S. Recent advances in C–H functionalization using electrochemical transition metal catalysis. ACS Catal. 8, 7179–7189 (2018).
Yang, Q.-L., Fang, P. & Mei, T.-S. Recent advances in organic electrochemical C–H functionalization. Chin. J. Chem. 36, 338–352 (2018).
Tang, S., Zeng, L. & Lei, A. Oxidative R1–H/R2–H cross-coupling with hydrogen evolution. J. Am. Chem. Soc. 140, 13128–13135 (2018).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Hou, Z.-W., Mao, Z.-Y. & Xu, H.-C. Recent progress on the synthesis of (aza)indoles through oxidative alkyne annulation reactions. Synlett 28, 1867–1872 (2017).
Yoshida, J., Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Jutand, A. Contribution of electrochemistry to organometallic catalysis. Chem. Rev. 108, 2300–2347 (2008).
Gandeepan, P. et al. 3d transition metals for C–H activation. Chem. Rev. 119, 2192–2452 (2019).
Loup, J., Dhawa, U., Pesciaioli, F., Wencel-Delord, J. & Ackermann, L. Enantioselective C–H activation with earth-abundant 3d transition metals. Angew. Chem. Int. Ed. 58, 12803–12818 (2019).
Khake, S. M. & Chatani, N. Chelation-assisted nickel-catalyzed C–H functionalizations. Trends Chem. 1, 524–539 (2019).
Zhu, X. & Chiba, S. Copper-catalyzed oxidative carbon–heteroatom bond formation: a recent update. Chem. Soc. Rev. 45, 4504–4523 (2016).
Liu, W. & Ackermann, L. Manganese-catalyzed C–H activation. ACS Catal. 6, 3743–3752 (2016).
Fürstner, A. Iron catalysis in organic synthesis: a critical assessment of what it takes to make this base metal a multitasking champion. ACS Cent. Sci. 2, 778–789 (2016).
Kalsi, D., Dutta, S., Barsu, N., Rueping, M. & Sundararaju, B. Room-temperature C–H bond functionalization by merging cobalt and photoredox catalysis. ACS Catal. 8, 8115–8120 (2018).
Grigorjeva, L. & Daugulis, O. Cobalt-catalyzed, aminoquinoline-directed C(sp2)–H bond alkenylation by alkynes. Angew. Chem. Int. Ed. 53, 10209–10212 (2014).
Gao, K. & Yoshikai, N. Low-valent cobalt catalysis: new opportunities for C–H functionalization. Acc. Chem. Res. 47, 1208–1219 (2014).
Song, W. & Ackermann, L. Cobalt-catalyzed direct arylation and benzylation by C–H/C–O cleavage with sulfamates, carbamates, and phosphates. Angew. Chem. Int. Ed. 51, 8251–8254 (2012).
Sauermann, N., Meyer, T. H., Tian, C. & Ackermann, L. Electrochemical cobalt-catalyzed C–H oxygenation at room temperature. J. Am. Chem. Soc. 139, 18452–18455 (2017).
Sauermann, N., Mei, R. & Ackermann, L. Electrochemical C–H amination by cobalt catalysis in a renewable solvent. Angew. Chem. Int. Ed. 57, 5090–5094 (2018).
Gao, X., Wang, P., Zeng, L., Tang, S. & Lei, A. Cobalt(II)-catalyzed electrooxidative C–H amination of arenes with alkylamines. J. Am. Chem. Soc. 140, 4195–4199 (2018).
Tian, C., Dhawa, U., Struwe, J. & Ackermann, L. Cobaltaelectro-catalyzed C–H acyloxylation. Chin. J. Chem. 37, 552–556 (2019).
Tian, C., Massignan, L., Meyer, T. H. & Ackermann, L. Electrochemical C–H/N–H activation by water-tolerant cobalt catalysis at room temperature. Angew. Chem. Int. Ed. 57, 2383–2387 (2018).
Mei, R., Sauermann, N., Oliveira, J. C. A. & Ackermann, L. Electroremovable traceless hydrazides for cobalt-catalyzed electro-oxidative C–H/N–H activation with internal alkynes. J. Am. Chem. Soc. 140, 7913–7921 (2018).
Tang, S., Wang, D., Liu, Y., Zeng, L. & Lei, A. Cobalt-catalyzed electrooxidative C–H/N–H [4+2] annulation with ethylene or ethyne. Nat. Commun. 9, 798 (2018).
Mei, R., Ma, W., Zhang, Y., Guo, X. & Ackermann, L. Cobaltaelectro-catalyzed oxidative C–H/N–H activation with 1,3-diynes by electro-removable hydrazides. Org. Lett. 21, 6534–6538 (2019).
Meyer, T. H., Oliveira, J. C. A., Sau, S. C., Ang, N. W. J. & Ackermann, L. Electrooxidative allene annulations by mild cobalt-catalyzed C–H activation. ACS Catal. 8, 9140–9147 (2018).
Sau, S. C., Mei, R., Struwe, J. & Ackermann, L. Cobaltaelectro-catalyzed C–H activation with carbon monoxide or isocyanides. ChemSusChem 12, 3023–3027 (2019).
Zeng, L. et al. Cobalt-catalyzed electrochemical oxidative C–H/N–H carbonylation with hydrogen evolution. ACS Catal. 8, 5448–5453 (2018).
Cardoso, D. S. P., Šljukić, B., Santos, D. M. F. & Sequeira, C. A. C. Organic electrosynthesis: from laboratorial practice to industrial applications. Org. Process Res. Dev. 21, 1213–1226 (2017).
Kong, W.-J. et al. Flow rhodaelectro-catalyzed alkyne annulations by versatile C–H activation: mechanistic support for rhodium(III/IV). J. Am. Chem. Soc. 141, 17198–17206 (2019).
Elsherbini, M. & Wirth, T. Electroorganic synthesis under flow conditions. Acc. Chem. Res. 52, 3287–3296 (2019).
Huang, C., Qian, X.-Y. & Xu, H.-C. Continuous-flow electrosynthesis of benzofused S-heterocycles by dehydrogenative C–S cross-coupling. Angew. Chem. Int. Ed. 58, 6650–6653 (2019).
Folgueiras-Amador, A. A., Philipps, K., Guilbaud, S., Poelakker, J. & Wirth, T. An easy-to-machine electrochemical flow microreactor: efficient synthesis of isoindolinone and flow functionalization. Angew. Chem. Int. Ed. 56, 15446–15450 (2017).
Schille, B., Giltzau, N. O. & Francke, R. On the use of polyelectrolytes and polymediators in organic electrosynthesis. Angew. Chem. Int. Ed. 57, 422–426 (2018).
Mei, R., Wang, H., Warratz, S., Macgregor, S. A. & Ackermann, L. Cobalt-catalyzed oxidase C–H/N–H alkyne annulation: mechanistic insights and access to anticancer agents. Chem. Eur. J. 22, 6759–6763 (2016).
Acknowledgements
Generous support by the DFG (Gottfried-Wilhelm-Leibniz award) to L.A., the CSC (scholarship to C.T.) and the DAAD (fellowship to U.D.) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
C.T., T.H.M. and L.A. designed the experiments. C.T., M.S., T.H.M., U.D. and K.R. performed the experiments. C.T., T.H.M., M.S. and L.H.F. designed the device. C.T., M.S., T.H.M., U.D. and L.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Fengzhi Zhang and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Mei, R. et al. J. Am. Chem. Soc. 140, 7913–7921 (2018): https://doi.org/10.1021/jacs.8b03521
Meyer, T. H. et al. ACS Catal. 8, 9140–9147 (2018): https://doi.org/10.1021/acscatal.8b03066
Tian, C. et al. Angew. Chem. Int. Ed. 57, 2383–2387 (2018): https://doi.org/10.1002/anie.201712647
Sauermann, N. et al. J. Am. Chem. Soc. 139, 18452–18455 (2017): https://doi.org/10.1021/jacs.7b11025
Key data used in this protocol
Tian, C. et al. Angew. Chem. Int. Ed. 57, 2383–2387 (2018): https://doi.org/10.1002/anie.201712647
Sauermann, N. et al. J. Am. Chem. Soc. 139, 18452–18455 (2017): https://doi.org/10.1021/jacs.7b11025
Integrated supplementary information
Supplementary Fig. 1 Cyclic voltammograms at 100 mVs–1.
n-Bu4NPF6 (0.1 M in MeOH), concentration of substrates 1 mM (NaOPiv 4 mM). (black) blank; (red) substrate 1a; (blue) Co(OAc)2∙4H2O and NaOPiv; (green) Co(OAc)2∙4H2O, NaOPiv and 1a.
Supplementary Fig. 2 Cyclic voltammograms at 100 mVs–1.
n-Bu4NPF6 (0.1 M in MeCN), concentration of substrates 1 mM (NaOPiv 4 mM). (black) blank; (red) substrate 1a; (blue) Co(OAc)2∙4H2O and NaOPiv; (green) Co(OAc)2∙4H2O, NaOPiv and 1a; (purple) Co(OAc)2∙4H2O, NaOPiv, 1a and EtOH (1 mM); (orange) Co(OAc)2∙4H2O, NaOPiv and 1a and EtOH (2 mM); (magenta) Co(OAc)2∙4H2O, NaOPiv and 1a and EtOH (4 mM).
Supplementary Fig. 3
Proposed catalytic cycle for cobaltaelectro-catalyzed oxygenation.
Supplementary Fig. 4
Proposed catalytic cycle for cobaltaelectro-catalyzed annulation.
Supplementary Fig. 5
Technical drawing of the thermal reservoir.
Supplementary Fig. 6
Schlenk-type glass cell.
Supplementary Fig. 7 Parameter settings of the galvanostat.
a) Set the maximum output voltage with “V-Set” to 5.000 V; b) Set the output current with “I-Set” to 0.0040 A; c) Press the “output” button to start the electrolysis.
Supplementary Fig. 8
Cleaning of the platinum electrode.
Supplementary Fig. 9
Gram-scale cobaltaelectrocatalysis setup.
Supplementary Fig. 10
Cobaltaelectrocatalysis in ElectraSyn 2.0.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Supplementary Methods.
Rights and permissions
About this article
Cite this article
Tian, C., Meyer, T.H., Stangier, M. et al. Cobaltaelectro-catalyzed C–H activation for resource-economical molecular syntheses. Nat Protoc 15, 1760–1774 (2020). https://doi.org/10.1038/s41596-020-0306-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0306-8
This article is cited by
-
Electrochemistry in organics: a powerful tool for “green” synthesis
Journal of Solid State Electrochemistry (2023)
-
Electrochemically time-dependent oxidative coupling/coupling-cyclization reaction between heterocycles: tunable synthesis of polycyclic indole derivatives with fluorescence properties
Science China Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.