Nature Chemistry ( IF 19.2 ) Pub Date : 2020-04-13 , DOI: 10.1038/s41557-020-0442-3 Robert C Godfrey 1 , Nicholas J Green 1 , Gary S Nichol 1 , Andrew L Lawrence 1
|
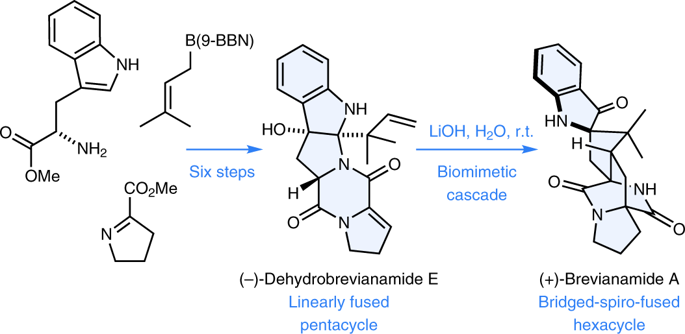
The fungal-derived bicyclo[2.2.2]diazaoctane alkaloids are of interest to the scientific community for their potent and varied biological activities. Within this large and diverse family of natural products, the insecticidal metabolite (+)-brevianamide A is particularly noteworthy for its synthetic intractability and inexplicable biogenesis. Despite five decades of research, this alkaloid has remained an elusive target for chemical synthesis due to insurmountable issues of reactivity and selectivity associated with all previously explored strategies. We herein report the chemical synthesis of (+)-brevianamide A (seven steps, 7.2% overall yield, 750 mg scale), which involves a bioinspired cascade transformation of the linearly fused (−)-dehydrobrevianamide E into the topologically complex bridged-spiro-fused structure of (+)-brevianamide A.

中文翻译:

灯盏花酰胺A的全合成
真菌衍生的双环[2.2.2]二氮杂辛烷生物碱因其强大而多样的生物活性而受到科学界的关注。在这个种类繁多的天然产物家族中,杀虫性代谢产物(+)-brevianamide A特别值得一提,因为它具有合成难治性和难以解释的生物发生机理。尽管进行了五十年的研究,由于与所有先前探索的策略相关的不可克服的反应性和选择性问题,该生物碱仍是化学合成的目标。我们在此报告了(+)-brevianamide A的化学合成(七个步骤,总收率7.2%,规模为750 mg),其中涉及线性融合的(-)-dehydrobrevianamide E到拓扑复杂的桥连螺环的生物启发级联转化。 (+)-brevianamide A的稠合结构












































 京公网安备 11010802027423号
京公网安备 11010802027423号