Abstract
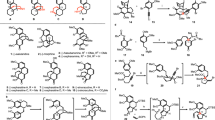
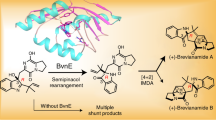
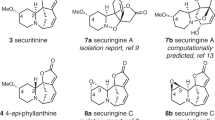
The fungal-derived bicyclo[2.2.2]diazaoctane alkaloids are of interest to the scientific community for their potent and varied biological activities. Within this large and diverse family of natural products, the insecticidal metabolite (+)-brevianamide A is particularly noteworthy for its synthetic intractability and inexplicable biogenesis. Despite five decades of research, this alkaloid has remained an elusive target for chemical synthesis due to insurmountable issues of reactivity and selectivity associated with all previously explored strategies. We herein report the chemical synthesis of (+)-brevianamide A (seven steps, 7.2% overall yield, 750 mg scale), which involves a bioinspired cascade transformation of the linearly fused (−)-dehydrobrevianamide E into the topologically complex bridged-spiro-fused structure of (+)-brevianamide A.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the characterization data and experimental protocols are provided in this article and its Supplementary Information. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 1918446 (compound 1). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Nicolaou, K. C., Snyder, S. A., Montagnon, T. & Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 41, 1668–1198 (2002).
Stocking, E. M. & Williams, R. M. Chemistry and biology of biosynthetic Diels–Alder reactions. Angew. Chem. Int. Ed. 42, 3078–3115 (2003).
Oikawa, H. & Tokiwano, T. Enzymatic catalysis of the Diels–Alder reaction in the biosynthesis of natural products. Nat. Prod. Rep. 21, 321–352 (2004).
Klas, K., Tsukamoto, S., Sherman, D. H. & Williams, R. M. Natural Diels–Alderases: elusive and irresistable. J. Org. Chem. 80, 11672–11685 (2015).
Minami, A. & Oikawa, H. Recent advances of Diels–Alderases involved in natural product biosynthesis. J. Antibiot. 69, 500–506 (2016).
Jamieson, C. S., Ohashi, M., Liu, F., Tang, Y. & Houk, K. N. The expanding world of biosynthetic pericyclases: cooperation of experiment and theory for discovery. Nat. Prod. Rep. 36, 698–713 (2019).
Finefield, J. M., Frisvad, J. C., Sherman, D. H. & Williams, R. M. Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids. J. Nat. Prod. 75, 812–833 (2012).
Klas, K. R. et al. Structural and stereochemical diversity in prenylated indole alkaloids containing the bicyclo[2.2.2]diazaoctane ring system from marine and terrestrial fungi. Nat. Prod. Rep. 35, 532–558 (2018).
Robertson, A. P. et al. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J. Pharmacol. Exp. Ther. 303, 853–860 (2002).
Little, P. R. et al. Efficacy of a combined oral formulation of derquantel-abamectin against the adult and larval stages of nematodes in sheep, including anthelmintic-resistant strains. Vet. Parasitol. 181, 180–193 (2011).
Buxton, S. K. et al. Investigation of acetylcholine receptor diversity in a nematode parasite leads to characterization of tribendimidine- and derquantel-sensitive nAChRs. PLoS Pathog. 10, e1003870 (2014).
Martínez-Luisa, S. et al. Malbrancheamide, a new calmodulin inhibitor from the fungus Malbranchea aurantiaca. Tetrahedron 62, 1817–1822 (2006).
Lin, Z. et al. Chrysogenamide A from an endophytic fungus associated with Cistanche deserticola and its neuroprotective effect on SH-SY5Y cells. J. Antibiot. 61, 81–85 (2008).
Qian-Cutrone, J. et al. Stephacidin A and B: two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 124, 14556–14557 (2002).
Kato, H. et al. Notoamides A–D: prenylated indole alkaloids isolated from a marine‐derived fungus, Aspergillus sp. Angew. Chem. Int. Ed. 46, 2254–2256 (2007).
Birch, A. J. & Wright, J. J. The brevianamides: a new class of fungal alkaloid. J. Chem. Soc. D 644–645 (1969).
Paterson, R. R. M., Simmonds, M. J. S., Kemmelmeier, C. & Blaney, W. M. Effects of brevianamide A, its photolysis product brevianamide D, and ochratoxin A from two Penicillium strains on the insect pests Spodoptera frugiperda and Heliothis virescens. Mycol. Res. 94, 538–542 (1990).
Bird, B. A. & Campbell, I. M. Brevianamides A and B are formed only after conidiation has begun in solid cultures of Penicillium brevicompactum. Appl. Environ. Microbiol. 42, 521–525 (1981).
Bird, B. A., Remaley, A. T. & Campbell, I. M. Disposition of mycophenolic acid, brevianamide A, asperphenamate, and ergosterol in solid cultures of Penicillium brevicompactum. Appl. Environ. Microbiol. 43, 345–348 (1982).
Porter, A. E. A. & Sammes, P. G. A Diels–Alder reaction of possible biosynthetic importance. J. Chem. Soc. D 1103a–1103a (1970).
Dan, Q. et al. Fungal indole alkaloid biogenesis through evolution of a bifunctional reductase/Diels–Alderase. Nat. Chem. 11, 972–980 (2019).
Li, S. et al. Comparative analysis of the biosynthetic systems for fungal bicyclo[2.2.2]diazaoctane indole alkaloids: the (+)/(−)-notoamide, paraherquamide and malbrancheamide pathways. Med. Chem. Commun. 3, 987–996 (2012).
Miller, K. A., Tsukamoto, S. & Williams, R. M. Asymmetric total syntheses of (+)- and (–)-versicolamide B and biosynthetic implications. Nat. Chem. 1, 68–69 (2009).
Williams, R. M., Glinka, T. & Kwast, E. Facial selectivity of the intramolecular SN2′ cyclization: stereocontrolled total synthesis of brevianamide B. J. Am. Chem. Soc. 110, 5927–5929 (1988).
Williams, R. M., Glinka, T., Kwast, E., Coffman, H. & Stille, J. K. Asymmetric, stereocontrolled total synthesis of (−)-brevianamide B. J. Am. Chem. Soc. 112, 808–821 (1990).
Williams, R. M., Sanz-Cervera, J. F., Sancenón, F., Marco, J. A. & Halligan, K. Biomimetic Diels−Alder cyclizations for the construction of the brevianamide, paraherquamide sclerotamide, and VM55599 ring systems. J. Am. Chem. Soc. 120, 1090–1091 (1998).
Williams, R. M., Sanz-Cervera, J. F., Sancenón, F., Marco, J. A. & Halligan, K. Biomimetic Diels–Alder cyclizations for the construction of the brevianamide, paraherquamide, sclerotamide, asperparaline and VM55599 ring systems. Bioorg. Med. Chem. 6, 1233–1241 (1998).
Adams, L. A., Valente, M. W. N. & Williams, R. M. A concise synthesis of d,l-brevianamide B via a biomimetically-inspired IMDA construction. Tetrahedron 62, 5195–5200 (2006).
Greshock, T. J. & Williams, R. M. Improved biomimetic total synthesis of d,l-stephacidin A. Org. Lett. 9, 4255–4258 (2007).
Frebault, F. C. & Simpkins, N. S. A cationic cyclisation route to prenylated indole alkaloids: synthesis of malbrancheamide B and brevianamide B, and progress towards stephacidin A. Tetrahedron 66, 6585–6596 (2010).
Robins, J. G., Kim, K. J., Chinn, A. J., Woo, J. S. & Scheerer, J. R. Intermolecular Diels−Alder cycloaddition for the construction of bicyclo[2.2.2]diazaoctane structures: formal synthesis of brevianamide B and premalbrancheamide. J. Org. Chem. 81, 2293–2301 (2016).
Perkins, J. C., Wang, X., Pike, R. D. & Scheerer, J. R. Further investigation of the intermolecular Diels–Alder cycloaddition for the synthesis of bicyclo[2.2.2]diazaoctane alkaloids. J. Org. Chem. 82, 13656–13662 (2017).
Williams, R. M., Kwast, E., Coffman, H. & Glinka, T. Remarkable, enantiodivergent biogenesis of brevianamide A and B. J. Am. Chem. Soc. 111, 3064–3065 (1989).
Birch, A. J. & Wright, J. J. Studies in relation to biosynthesis—XLII: the structural elucidation and some aspects of the biosynthesis of the brevianamides-A and -E. Tetrahedron 26, 2329–2344 (1970).
Birch, A. J. & Russell, R. A. Studies in relation to biosynthesis—XLIV: structural elucidations of brevianamides-B, -C, -D and -F. Tetrahedron 28, 2999–3008 (1972).
Baldas, J., Birch, A. J. & Russell, R. A. Studies in relation to biosynthesis. Part XLVI. Incorporation of cyclo-l-tryptophyl-l-proline into brevianamide A. J. Chem. Soc. Perkin Trans. 1, 50–52 (1974).
Sanz-Cervera, J. F., Glinka, T. & Williams, R. M. Biosynthesis of brevianamides A and B: in search of the biosynthetic Diels-Alder construction. Tetrahedron 49, 8471–8482 (1993).
Domingo, L. R., Sanz-Cervera, J. F., Williams, R. M., Picher, M. T. & Marco, J. A. Biosynthesis of the brevianamides. An ab initio study of the biosynthetic intramolecular Diels–Alder cycloaddition. J. Org. Chem. 62, 1662–1667 (1997).
Steyn, P. S. The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron 29, 107–120 (1973).
Scott, P. M., Kennedy, B. P. C., Harwig, J. & Chen, Y.-K. Formation of diketopiperazines by Penicillium italicum isolated from oranges. Appl. Microbiol 28, 892–894 (1974).
Kaur, A., Raja, H. A., Deep, G., Agarwal, R. & Oberlies, N. H. Pannorin B, a new naphthopyrone from an endophytic fungal isolate of Penicillium sp. Magn. Reson. Chem. 54, 164–167 (2016).
Liu, H., Pattabiraman, V. R. & Vederas, J. C. Stereoselective syntheses of 4-oxa diaminopimelic acid and its protected derivatives via aziridine ring opening. Org. Lett. 9, 4211–4214 (2007).
Schkeryantz, J. M., Woo, J. C. G., Siliphaivanh, P., Depew, K. M. & Danishefsky, S. J. Total synthesis of gypsetin, deoxybrevianamide E, brevianamide E, and tryprostatin B: novel constructions of 2,3-disubstituted indoles. J. Am. Chem. Soc. 121, 11964–11975 (1999).
Zhao, L., May, J. P., Huang, J. & Perrin, D. M. Stereoselective synthesis of brevianamide E. Org. Lett. 14, 90–93 (2012).
Fisher, J. W. & Trinkle, K. L. Iodide dealkylation of benzyl, PMB, PNB, and t-butyl N-acyl amino acid esters via lithium ion coordination. Tetrahedron Lett. 35, 2505–2508 (1994).
Huy, P., Neudörfl, J.-M. & Schmalz, H.-G. A practical synthesis of trans-3-substituted proline derivatives through 1,4-addition. Org. Lett. 13, 216–219 (2011).
Nigst, T. A., Antipova, A. & Mayr, H. Nucleophilic reactivities of hydrazines and amines: the futile search for the α-effect in hydrazine reactivities. J. Org. Chem. 77, 8142–8155 (2012).
Kametani, T., Kanaya, N. & Ihara, M. Asymmetric total synthesis of brevianamide E. J. Am. Chem. Soc. 102, 3974–3975 (1980).
Ye, Y. et al. Cofactor-independent pinacolase directs non-Diels–Alderase biogenesis of the brevianamides. Preprint at https://doi.org/10.26434/chemrxiv.9122009.v1 (2019).
Acknowledgements
This work was supported by an EPSRC First Grant (EP/N029542/1) and a Marie Curie Career Integration Grant (631132, POSIN). We thank T. Herlt for assistance and advice regarding chromatography, and acknowledge SIRCAMS at the University of Edinburgh for mass spectrometry.
Author information
Authors and Affiliations
Contributions
R.C.G., N.J.G. and A.L.L. conceived, designed and carried out the synthetic experiments. G.S.N. performed the crystallographic studies. All authors discussed and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Overview of previous total and formal syntheses of brevianamide B, Supplementary Tables 1–3, details of the materials and methods, experimental procedures and compound characterization data (1H NMR,13C NMR, IR, HRMS, optical rotations, chiral-HPLC and X-ray).
Crystallographic data
Crystallographic data for compound 1. CCDC 1918446.
Rights and permissions
About this article
Cite this article
Godfrey, R.C., Green, N.J., Nichol, G.S. et al. Total synthesis of brevianamide A. Nat. Chem. 12, 615–619 (2020). https://doi.org/10.1038/s41557-020-0442-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0442-3
This article is cited by
-
One-pot synthesis of 2,5-diketopiperazine with high titer and versatility using adenylation enzyme
Applied Microbiology and Biotechnology (2022)
-
Fungal-derived brevianamide assembly by a stereoselective semipinacolase
Nature Catalysis (2020)