Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-04-06 , DOI: 10.1038/s41594-020-0402-z Aaron P Owji 1 , Qingqing Zhao 2, 3 , Changyi Ji 2 , Alec Kittredge 2 , Austin Hopiavuori 2 , Ziao Fu 4 , Nancy Ward 2 , Oliver B Clarke 5 , Yin Shen 3 , Yu Zhang 6 , Wayne A Hendrickson 4, 5, 7 , Tingting Yang 2, 6
|
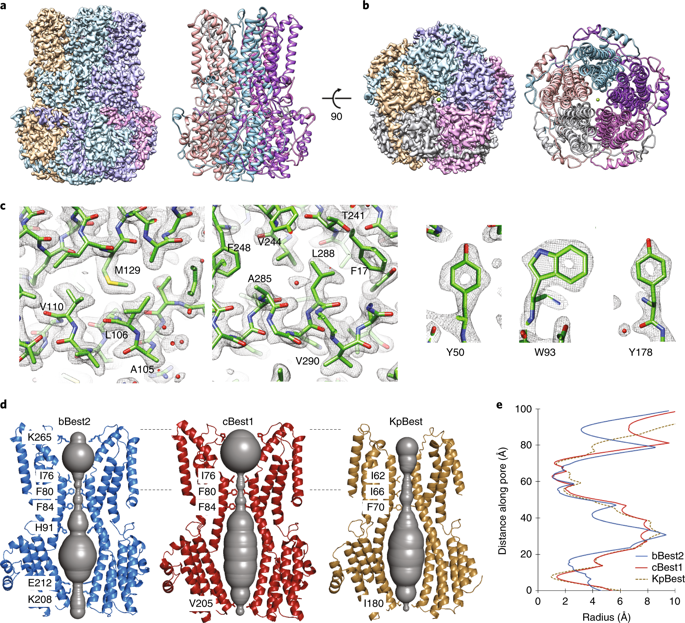
The bestrophin family of calcium (Ca2+)-activated chloride (Cl−) channels, which mediate the influx and efflux of monovalent anions in response to the levels of intracellular Ca2+, comprises four members in mammals (bestrophin 1–4). Here we report cryo-EM structures of bovine bestrophin-2 (bBest2) bound and unbound by Ca2+ at 2.4- and 2.2-Å resolution, respectively. The bBest2 structure highlights four previously underappreciated pore-lining residues specifically conserved in Best2 but not in Best1, illustrating the differences between these paralogs. Structure-inspired electrophysiological analysis reveals that, although the channel is sensitive to Ca2+, it has substantial Ca2+-independent activity for Cl−, reflecting the opening at the cytoplasmic restriction of the ion conducting pathway even when Ca2+ is absent. Moreover, the ion selectivity of bBest2 is controlled by multiple residues, including those involved in gating.
中文翻译:

bestropin-2 阴离子通道的结构和功能表征。
钙 (Ca 2+ ) 激活氯 (Cl − ) 通道的 bestropin 家族可根据细胞内 Ca 2+水平介导单价阴离子的流入和流出,在哺乳动物中由四个成员组成(bestropin 1–4) 。在这里,我们分别以 2.4-Å 和 2.2-Å 分辨率报道了与 Ca 2+结合和未结合的牛 bestropin-2 (bBest2) 的冷冻电镜结构。 bBest2 结构突出显示了 Best2 中特别保守但 Best1 中不保守的四个先前未被充分认识的孔衬残基,说明了这些旁系同源物之间的差异。结构启发的电生理学分析表明,尽管该通道对 Ca 2+敏感,但它对 Cl -具有显着的与 Ca 2+无关的活性,这反映了即使在 Ca 2+不存在时,离子传导通路的细胞质限制也是开放的。此外,bBest2 的离子选择性由多个残基控制,包括参与门控的残基。









































 京公网安备 11010802027423号
京公网安备 11010802027423号