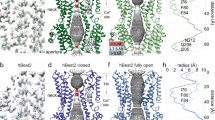
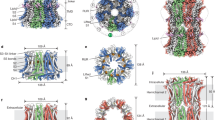
Abstract
The bestrophin family of calcium (Ca2+)-activated chloride (Cl−) channels, which mediate the influx and efflux of monovalent anions in response to the levels of intracellular Ca2+, comprises four members in mammals (bestrophin 1–4). Here we report cryo-EM structures of bovine bestrophin-2 (bBest2) bound and unbound by Ca2+ at 2.4- and 2.2-Å resolution, respectively. The bBest2 structure highlights four previously underappreciated pore-lining residues specifically conserved in Best2 but not in Best1, illustrating the differences between these paralogs. Structure-inspired electrophysiological analysis reveals that, although the channel is sensitive to Ca2+, it has substantial Ca2+-independent activity for Cl−, reflecting the opening at the cytoplasmic restriction of the ion conducting pathway even when Ca2+ is absent. Moreover, the ion selectivity of bBest2 is controlled by multiple residues, including those involved in gating.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hartzell, H. C., Qu, Z., Yu, K., Xiao, Q. & Chien, L. T. Molecular physiology of bestrophins: multifunctional membrane proteins linked to Best disease and other retinopathies. Physiol. Rev. 88, 639–672 (2008).
Xiao, Q., Hartzell, H. C. & Yu, K. Bestrophins and retinopathies. Pflugers Arch. 460, 559–569 (2010).
Sun, H., Tsunenari, T., Yau, K. W. & Nathans, J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl Acad. Sci. USA 99, 4008–4013 (2002).
Tsunenari, T. et al. Structure-function analysis of the bestrophin family of anion channels. J. Biol. Chem. 278, 41114–41125 (2003).
Marmorstein, A. D. et al. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl Acad. Sci. USA 97, 12758–12763 (2000).
Marquardt, A. et al. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best’s disease). Hum. Mol. Genet. 7, 1517–1525 (1998).
Petrukhin, K. et al. Identification of the gene responsible for Best macular dystrophy. Nat. Genet. 19, 241–247 (1998).
Allikmets, R. et al. Evaluation of the Best disease gene in patients with age-related macular degeneration and other maculopathies. Hum. Genet. 104, 449–453 (1999).
Burgess, R. et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am. J. Hum. Genet. 82, 19–31 (2008).
Davidson, A. E. et al. Missense mutations in a retinal pigment epithelium protein, bestrophin-1, cause retinitis pigmentosa. Am. J. Hum. Genet. 85, 581–592 (2009).
Ji, C. et al. Investigation and restoration of BEST1 activity in patient-derived RPEs with dominant mutations. Sci. Rep. 9, 19026 (2019).
Kramer, F. et al. Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (Best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. Eur J. Hum. Genet. 8, 286–292 (2000).
Yang, T., Justus, S., Li, Y. & Tsang, S. H. BEST1: the Best target for gene and cell therapies. Mol. Ther. 23, 1805–1809 (2015).
Yardley, J. et al. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC). Invest. Ophthalmol. Vis. Sci. 45, 3683–3689 (2004).
Bakall, B. et al. Bestrophin-2 is involved in the generation of intraocular pressure. Invest. Ophthalmol. Vis. Sci. 49, 1563–1570 (2008).
Zhang, Y., Patil, R. V. & Marmorstein, A. D. Bestrophin 2 is expressed in human non-pigmented ciliary epithelium but not retinal pigment epithelium. Mol. Vis. 16, 200–206 (2010).
Heijl, A., Leske, M. C., Bengtsson, B., Hyman, L. & Hussein, M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120, 1268–1279 (2002).
Kass, M. A. et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120, 701–713 (2002).
Lichter, P. R. et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 108, 1943–1953 (2001).
Cui, C. Y. et al. Forkhead transcription factor FoxA1 regulates sweat secretion through Bestrophin 2 anion channel and Na-K-Cl cotransporter 1. Proc. Natl Acad. Sci. USA 109, 1199–1203 (2012).
Yu, K., Lujan, R., Marmorstein, A., Gabriel, S. & Hartzell, H. C. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J. Clin. Invest. 120, 1722–1735 (2010).
Kane Dickson, V., Pedi, L. & Long, S. B. Structure and insights into the function of a Ca2+ -activated Cl− channel. Nature 516, 213–218 (2014).
Yang, T. et al. Structure and selectivity in bestrophin ion channels. Science 346, 355–359 (2014).
Vaisey, G., Miller, A. N. & Long, S. B. Distinct regions that control ion selectivity and calcium-dependent activation in the bestrophin ion channel. Proc. Natl Acad. Sci. USA 113, E7399–E7408 (2016).
Zhang, Y. et al. ATP activates bestrophin ion channels through direct interaction. Nat. Commun. 9, 3126 (2018).
Qu, Z., Chien, L. T., Cui, Y. & Hartzell, H. C. The anion-selective pore of the bestrophins, a family of chloride channels associated with retinal degeneration. J. Neurosci. 26, 5411–5419 (2006).
Qu, Z., Fischmeister, R. & Hartzell, C. Mouse bestrophin-2 is a bona fide Cl− channel: identification of a residue important in anion binding and conduction. J. Gen Physiol. 123, 327–340 (2004).
Qu, Z. & Hartzell, C. Determinants of anion permeation in the second transmembrane domain of the mouse bestrophin-2 chloride channel. J. Gen. Physiol. 124, 371–382 (2004).
Li, Y. et al. Patient-specific mutations impair BESTROPHIN1’s essential role in mediating Ca2+-dependent Cl− currents in human RPE. Elife 6, e29914 (2017).
Qu, Z., Wei, R. W., Mann, W. & Hartzell, H. C. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl− currents. J. Biol. Chem. 278, 49563–49572 (2003).
Ji, C. et al. Dual Ca2+-dependent gates in human Bestrophin1 underlie disease-causing mechanisms of gain-of-function mutations. Commun. Biol. 2, 240 (2019).
Miller, A. N., Vaisey, G. & Long, S. B. Molecular mechanisms of gating in the calcium-activated chloride channel bestrophin. Elife 8, e43231 (2019).
Civan, M. M., Peterson-Yantorno, K., Sanchez-Torres, J. & Coca-Prados, M. Potential contribution of epithelial Na+ channel to net secretion of aqueous humor. J. Exp. Zool. 279, 498–503 (1997).
Jacob, T. J. & Civan, M. M. Role of ion channels in aqueous humor formation. Am. J. Physiol. 271, C703–C720 (1996).
Qu, Z. & Hartzell, H. C. Bestrophin Cl− channels are highly permeable to HCO3 −. Am. J. Physiol. Cell Physiol. 294, C1371–C1377 (2008).
Frings, S., Reuter, D. & Kleene, S. J. Neuronal Ca2+-activated Cl− channels–homing in on an elusive channel species. Prog. Neurobiol. 60, 247–289 (2000).
Kittredge, A., Ward, N., Hopiavuori, A., Zhang, Y. & Yang, T. Expression and purification of mammalian bestrophin ion channels. J. Vis. Exp. 138, e57832 (2018).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Rice, W. J. et al. Routine determination of ice thickness for cryo-EM grids. J. Struct. Biol. 204, 38–44 (2018).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Preprint at bioRxiv https://doi.org/10.1101/845032 (2020).
Heymann, J. B. Guidelines for using Bsoft for high resolution reconstruction and validation of biomolecular structures from electron micrographs. Protein Sci. 27, 159–171 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. Elife 5, e18722 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Asarnow, D., Palovcak, E. & Chen, Y. asarnow/pyem: UCSF pyem v.0.5. (UCSF, 2019).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Benkert, P., Biasini, M. & Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27, 343–350 (2011).
Bertoni, M., Kiefer, F., Biasini, M., Bordoli, L. & Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 7, 10480 (2017).
Bienert, S. et al. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 45, D313–D319 (2017).
Guex, N., Peitsch, M. C. & Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30, S162–S173 (2009).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21 (2010).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D. Struct. Biol. 74, 814–840 (2018).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 8, e1002708 (2012).
Acknowledgements
We thank the Center on Membrane Protein Production and Analysis (New York Structural Biology Center, supported by the National Institutes of Health (NIH) grant no. GM116799) and A. Sobolevsky for help on mammalian bestrophin screening; E. Eng for help on collecting cryo-EM data and R. Grassucci for assistance with microscope operation for initial screening experiments. Cryo-EM data were collected at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy (New York Structural Biology Center), supported by grants from the Simons Foundation (no. 349247), NYSTAR and NIH (no. GM103310). A.P.O. was supported by NIH grant no. (F31) EY030763, Y.S. was supported by the National Key R&D Program of China (2017YFE0103400), W.A.H. was supported by NIH grant no. GM107462 and T.Y. was supported by NIH grant nos. EY025290 and GM127652 and Columbia University start-up funding.
Author information
Authors and Affiliations
Contributions
A.P.O. designed research, performed protein purification and cryo-EM experiments, analyzed data, made figures and helped with writing the paper. Q.Z. and C.J. performed and analyzed patch clamp recordings, A.K. made constructs and purified proteins, A.H. maintained cells, Z.F. helped with collecting and analyzing cryo-EM data and N.W. made viruses. O.B.C. helped with cryo-EM data processing. Y.S. supervised Q.Z. and edited the manuscript. Y.Z. screened and purified proteins and wrote the paper. W.A.H. designed research and analyzed data. T.Y. designed research, analyzed data, made figures and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Peer review information Katarzyna Marcinkiewicz and Ines Chen were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Structure determination of bBest2 with 250 nM Ca2+.
a, b, FSC curve for masked and unmasked map generated by cryoSPARC v2 non-uniform refinement for Ca2+-bound (a) and Ca2+-unbound (b) states. c, d, Local resolution estimation calculated by blocres as implemented in cryoSPARC v2 for Ca2+-bound (c) and Ca2+-unbound (d) states. e, Viewing angles for particles contributing to final map as implemented by pyem star2bild script for Ca2+-bound state. f, Analysis of sphericity of final maps by 3DFSC server to assess directional anisotropy of specimens with slightly preferred orientation for Ca2+-bound state. g, h, The same format for the Ca2+-unbound state. Blue histogram indicates percentage of per angle FSC. Red solid line indicates directional FSC and green dashed line indicates + /− 1 standard deviation from mean of directional FSC. i, j, Map v. Model FSC curve with and without mask as implemented by Phenix.validation package for Ca2+-bound (i) and Ca2+-unbound (j) states.
Extended Data Fig. 2 Structure determination of bBest2 with EGTA only.
In the same format as Extended Data Fig. 2. Left (a, c, e, f, i), Ca2+-unbound state 1 (N-terminus partially disordered); right (b, d, g, h, j), Ca2+-unbound state 2 (N-terminus completely disordered).
Extended Data Fig. 4 Representative cryo-EM density for the two bBest2 cryo-EM structures with 250 nM Ca2+.
a, Architecture of an individual bBest2 protomer (left) and cBest1 protomer (4RDQ, right), both Ca2+-bound and color-coded by segments in accordance with Supplementary Figure 1. b–h, Representative map densities for indicated regions are shown with the corresponding atomic model. The Ca2+-bound state (ordered N-terminus) and Ca2+-unbound state (completely disordered N-terminus) structures are shown in orange (left) and blue (right), respectively. Green dot, a Ca2+ ion. i, j, Stereo images of bBest2, presenting the aperture region for divergent “wall-eyed” viewing. Orange, Ca2+-bound (i); blue, Ca2+-unbound (j). Green dot, a Cl- ion.
Extended Data Fig. 5 Representative cryo-EM density for bBest2 cryo-EM structures with EGTA only and 5 mM Ca2+.
a–g, Representative map densities for indicated regions are shown with the corresponding atomic model. The Ca2+-unbound state 1 (partially disordered N-terminus, EGTA only), Ca2+-unbound state 2 (completely disordered N-terminus, EGTA only) and Ca2+-bound state (ordered N-terminus, 5 mM Ca2+) structures are shown in green (left), pumpkin (middle) and purple (right), respectively. Green dot, a Ca2+ ion.
Extended Data Fig. 6 Critical residues in Best1 and Best2.
a, b, Population steady-state current density-voltage relationships from HEK293 cells expressing hBest2 S205I (a) or bBest2 G205I (b) in the absence (gray) and presence (black) of 1.2 μM [Ca2+]i; n = 9–20 for each point. c, Bar chart showing the steady-state current densities from HEK293 cells expressing the indicated Best1 and Best2 channels in the absence (gray) and presence (black) of 1.2 μM [Ca2+]i, n = 5–20 for each bar. *P < 0.05 compared to currents without Ca2+ under the same condition, using two-tailed unpaired Student t test. d, e, Population steady-state current density-voltage relationships from HEK293 cells expressing WT bBest2 in the presence of 1.2 μM [Ca2+]i with 120 mM NaCl in the internal solution, and 30 mM NaCl (d) or 120 mM NaSCN (e) in the external solution; n = 5 for each point. f, Population steady-state current density-voltage relationships from HEK293 cells expressing WT bBest2 in the presence of 1.2 μM [Ca2+]i with 120 and 30 mM NaCl in the internal and external solutions, respectively. Results from solutions buffered with NMDG (open circle) were compared to those from solutions buffered with NaOH (solid circle, the same data set as in d); n = 5–6 for each point. g, Population steady-state current density-voltage relationships from HEK293 cells expressing WT bBest2 in the presence of 1.2 μM [Ca2+]i with 120 mM NMDG-Cl and NMDG-CH3SO3 in the internal and external solutions, respectively (open circle), compared to results from 120 mM NaCl and NaCH3SO3 in the internal and external solutions, respectively (solid circle); n = 5–6 for each point. h, Reversal potentials from HEK293 cells expressing bBest2 WT, 3A, H91A, G199A, K208A and K265A. n = 5–18 for each point, the same color labels as in Fig. 3d. The graphs are plotted using the same set of data as in Figs. 3 and 7. All error bars in this figure represent s.e.m.
Extended Data Fig. 7 Expression and membrane trafficking of bBest2 mutants.
a, Top, Western blot showing the transient expression levels of bBest2 WT and mutants in HEK293 whole-cell lysate; Bottom, β-Actin loading control from the same gel. b, Top, cell surface expression levels of bBest2 WT and mutants in HEK293 cells were detected by immunoblotting. Membrane extractions were generated from the same batch of cell pellets as in a; Bottom, quantitation of the levels of bBest2 in plasma membrane from three independent experiments. Data were normalized to the global bBest2 level and then compared to WT. All error bars in this figure represent s.e.m.
Extended Data Fig. 8 A two-gate activation model of bBest2.
a–c, Cartoon showing the effect of Ca2+ on the permeation of Cl- (a), CH3SO3− (b) and I− (c). The number indicates the radius (Å) of the dehydrated anion. The thickness of the arrows reflect conductance of the channel.
Extended Data Fig. 9 A putative anion binding site within the aperture of bBest2.
Left, the molecular model of bBest2. Right, representative map densities with the corresponding atomic model for EGTA Ca2+-unbound state 1 (green), EGTA Ca2+-unbound state 2 (pumpkin), and 5 mM Ca2+-bound state (purple). a, Map processed with C5 symmetry, view from inside the channel towards the aperture, as depicted in the ribbon cartoon (left). b, Map processed with C5 symmetry, view from the side of the aperture, sliced to exclude one protomer, as depicted in the ribbon cartoon (left). c, Map processed with C1 symmetry using the same particle set as its respective C5 map above, same view as in a.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Owji, A.P., Zhao, Q., Ji, C. et al. Structural and functional characterization of the bestrophin-2 anion channel. Nat Struct Mol Biol 27, 382–391 (2020). https://doi.org/10.1038/s41594-020-0402-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0402-z
This article is cited by
-
Structures and gating mechanisms of human bestrophin anion channels
Nature Communications (2022)
-
Bestrophin-2 and glutamine synthetase form a complex for glutamate release
Nature (2022)
-
Cryo-EM structures of thylakoid-located voltage-dependent chloride channel VCCN1
Nature Communications (2022)
-
Rhodopsin-bestrophin fusion proteins from unicellular algae form gigantic pentameric ion channels
Nature Structural & Molecular Biology (2022)
-
Cycloalkane-modified amphiphilic polymers provide direct extraction of membrane proteins for CryoEM analysis
Communications Biology (2021)