当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directional conformer exchange in dihydrofolate reductase revealed by single-molecule nanopore recordings.
Nature Chemistry ( IF 19.2 ) Pub Date : 2020-04-06 , DOI: 10.1038/s41557-020-0437-0 Nicole Stéphanie Galenkamp 1 , Annemie Biesemans 2 , Giovanni Maglia 1, 2
Nature Chemistry ( IF 19.2 ) Pub Date : 2020-04-06 , DOI: 10.1038/s41557-020-0437-0 Nicole Stéphanie Galenkamp 1 , Annemie Biesemans 2 , Giovanni Maglia 1, 2
Affiliation
|
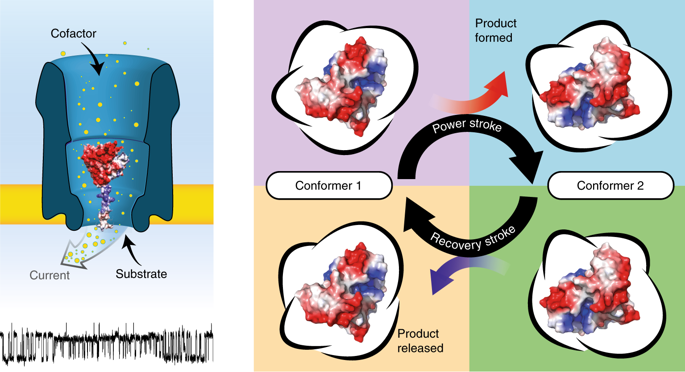
Conformational heterogeneity is emerging as a defining characteristic of enzyme function. However, understanding the role of protein conformations requires their thermodynamic and kinetic characterization at the single-molecule level, which remains extremely challenging. Here we report the ligand-induced conformational changes of dihydrofolate reductase (DHFR) by measuring the modulation of the nanopore currents. The long observation time of the electrical recordings enabled the detection of rare conformational transitions hidden in ensemble measurements. We show that DHFR exists in at least four ground-state configurations or conformers with different affinities for its ligands. Unliganded DHFR adopted low-affinity conformers, whereas the binding of substrates promoted the switch to the high-affinity conformer. Conversion between the conformers was accelerated by molecules that stabilized the transition state of DHFR, which suggests that the reaction lowers the energy barrier for conformer exchange and thus facilitates product release. This mechanism might be a general feature in enzymatic reactions affected by product inhibition or when the release of products is the rate-limiting step.
中文翻译:

单分子纳米孔记录揭示了二氢叶酸还原酶中的定向构象异构体交换。
构象异质性正在成为酶功能的决定性特征。但是,了解蛋白质构象的作用需要在单分子水平上对其进行热力学和动力学表征,这仍然极具挑战性。在这里,我们通过测量纳米孔电流的调制报告二氢叶酸还原酶(DHFR)的配体诱导的构象变化。电记录的长观察时间使得能够检测出隐藏在整体测量中的稀有构象转变。我们表明DHFR存在于至少四个基态配置或与其配体具有不同亲和力的构象异构体。未配体的DHFR采用低亲和力构象体,而底物的结合促进了向高亲和力构象体的转换。稳定DHFR过渡态的分子促进了构象异构体之间的转化,这表明该反应降低了构象异构体交换的能垒,从而促进了产物的释放。该机制可能是受产物抑制影响的酶促反应的一般特征,或者当产物的释放是限速步骤时。
更新日期:2020-04-24
中文翻译:

单分子纳米孔记录揭示了二氢叶酸还原酶中的定向构象异构体交换。
构象异质性正在成为酶功能的决定性特征。但是,了解蛋白质构象的作用需要在单分子水平上对其进行热力学和动力学表征,这仍然极具挑战性。在这里,我们通过测量纳米孔电流的调制报告二氢叶酸还原酶(DHFR)的配体诱导的构象变化。电记录的长观察时间使得能够检测出隐藏在整体测量中的稀有构象转变。我们表明DHFR存在于至少四个基态配置或与其配体具有不同亲和力的构象异构体。未配体的DHFR采用低亲和力构象体,而底物的结合促进了向高亲和力构象体的转换。稳定DHFR过渡态的分子促进了构象异构体之间的转化,这表明该反应降低了构象异构体交换的能垒,从而促进了产物的释放。该机制可能是受产物抑制影响的酶促反应的一般特征,或者当产物的释放是限速步骤时。











































 京公网安备 11010802027423号
京公网安备 11010802027423号