当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regioselective hydroarylation and arylation of maleimides with indazoles via a Rh(iii)-catalyzed C-H activation.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-29 , DOI: 10.1039/d0ob00353k Asim Kumar Ghosh 1 , Sadhanendu Samanta 1 , Payel Ghosh 1 , Sukanya Neogi 1 , Alakananda Hajra 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-29 , DOI: 10.1039/d0ob00353k Asim Kumar Ghosh 1 , Sadhanendu Samanta 1 , Payel Ghosh 1 , Sukanya Neogi 1 , Alakananda Hajra 1
Affiliation
|
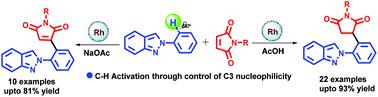
Switchable Rh(iii)-catalyzed highly regioselective hydroarylation and oxidative arylation of maleimides with 2-arylindazoles via C-H activation have been demonstrated. The reaction affords 3-(2-(2H-indazol-2-yl)phenyl)succinimide and 3-(2-(2H-indazol-2-yl)phenyl)maleimide derivatives in high yields with wide functional group tolerance. A mechanistic study was performed to depict C-H bond cleavage that might be involved in the turnover limiting step.
中文翻译:

通过Rh(iii)催化的CH活化,马来酰亚胺与吲唑的区域选择性加氢芳基化和芳基化。
已经证明可切换的Rh(iii)催化的高区域选择性加氢芳基化和马来酰亚胺与2-芳基吲唑的CH活化氧化芳基化反应。该反应以高收率和宽的官能团耐受性得到3-(2-(2H-吲唑-2-基)苯基)琥珀酰亚胺和3-(2-(2H-吲唑-2-基)苯基)马来酰亚胺衍生物。进行了一项机械学研究,以描述可能限制营业额的步骤涉及的CH键裂解。
更新日期:2020-03-30
中文翻译:

通过Rh(iii)催化的CH活化,马来酰亚胺与吲唑的区域选择性加氢芳基化和芳基化。
已经证明可切换的Rh(iii)催化的高区域选择性加氢芳基化和马来酰亚胺与2-芳基吲唑的CH活化氧化芳基化反应。该反应以高收率和宽的官能团耐受性得到3-(2-(2H-吲唑-2-基)苯基)琥珀酰亚胺和3-(2-(2H-吲唑-2-基)苯基)马来酰亚胺衍生物。进行了一项机械学研究,以描述可能限制营业额的步骤涉及的CH键裂解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号