当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
X-ray Structure of the Human Karyopherin RanBP5, an Essential Factor for Influenza Polymerase Nuclear Trafficking.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.jmb.2020.03.021 Christopher Swale 1 , Bruno Da Costa 2 , Laura Sedano 2 , Frédéric Garzoni 3 , Andrew A McCarthy 3 , Imre Berger 4 , Christoph Bieniossek 5 , Rob W H Ruigrok 6 , Bernard Delmas 2 , Thibaut Crépin 6
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.jmb.2020.03.021 Christopher Swale 1 , Bruno Da Costa 2 , Laura Sedano 2 , Frédéric Garzoni 3 , Andrew A McCarthy 3 , Imre Berger 4 , Christoph Bieniossek 5 , Rob W H Ruigrok 6 , Bernard Delmas 2 , Thibaut Crépin 6
Affiliation
|
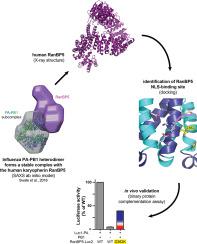
Here, we describe the crystal structures of two distinct isoforms of ligand-free human karyopherin RanBP5 and investigate its global propensity to interact with influenza A virus polymerase. Our results confirm the general architecture and mechanism of the IMB3 karyopherin-β subfamily whilst also highlighting differences with the yeast orthologue Kap121p. Moreover, our results provide insight into the structural flexibility of β-importins in the unbound state. Based on docking of a nuclear localisation sequence, point mutations were designed, which suppress influenza PA-PB1 subcomplex binding to RanBP5 in a binary protein complementation assay.
中文翻译:

人类核球蛋白RanBP5的X射线结构,这是流感聚合酶核贩运的重要因素。
在这里,我们描述了无配体的人类核球蛋白RanBP5的两个不同同工型的晶体结构,并研究了其与甲型流感病毒聚合酶相互作用的总体趋势。我们的研究结果证实了IMB3karyopherin-β亚家族的总体结构和机制,同时也强调了与酵母直向同源Kap121p的差异。此外,我们的结果提供了对未结合状态下β-importins的结构灵活性的见解。基于核定位序列的对接,设计了点突变,该突变在二元蛋白互补测定中抑制了流感PA-PB1亚复合体与RanBP5的结合。
更新日期:2020-03-25
中文翻译:

人类核球蛋白RanBP5的X射线结构,这是流感聚合酶核贩运的重要因素。
在这里,我们描述了无配体的人类核球蛋白RanBP5的两个不同同工型的晶体结构,并研究了其与甲型流感病毒聚合酶相互作用的总体趋势。我们的研究结果证实了IMB3karyopherin-β亚家族的总体结构和机制,同时也强调了与酵母直向同源Kap121p的差异。此外,我们的结果提供了对未结合状态下β-importins的结构灵活性的见解。基于核定位序列的对接,设计了点突变,该突变在二元蛋白互补测定中抑制了流感PA-PB1亚复合体与RanBP5的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号