Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NCOA5 deficiency promotes a unique liver protumorigenic microenvironment through p21WAF1/CIP1 overexpression, which is reversed by metformin.
Oncogene ( IF 6.9 ) Pub Date : 2020-03-20 , DOI: 10.1038/s41388-020-1256-x Mark Williams 1, 2 , Xinhui Liu 1, 3, 4 , Yueqi Zhang 1, 2 , Jake Reske 1 , Devika Bahal 5 , Trevor G Gohl 1, 6 , Daniel Hollern 7 , Elliot Ensink 1 , Matti Kiupel 8 , Rongcheng Luo 3 , Rupali Das 1 , Hua Xiao 1
Oncogene ( IF 6.9 ) Pub Date : 2020-03-20 , DOI: 10.1038/s41388-020-1256-x Mark Williams 1, 2 , Xinhui Liu 1, 3, 4 , Yueqi Zhang 1, 2 , Jake Reske 1 , Devika Bahal 5 , Trevor G Gohl 1, 6 , Daniel Hollern 7 , Elliot Ensink 1 , Matti Kiupel 8 , Rongcheng Luo 3 , Rupali Das 1 , Hua Xiao 1
Affiliation
|
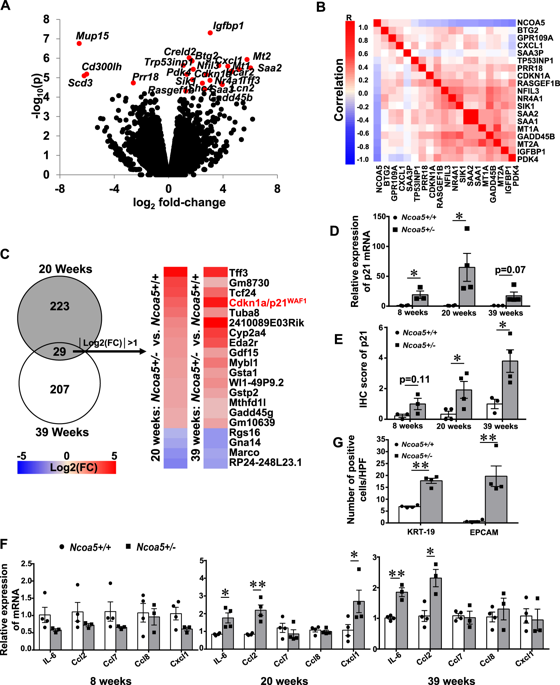
Prevention and treatment options for hepatocellular carcinoma (HCC) are presently limited, underscoring the necessity for further elucidating molecular mechanisms underlying HCC development and identifying new prevention and therapeutic targets. Here, we demonstrate a unique protumorigenic niche in the livers of Ncoa5+/- mouse model of HCC, which is characterized by altered expression of a subset of genes including p21WAF1/CIP1 and proinflammatory cytokine genes, increased putative hepatic progenitors, and expansions of activated and tissue-resident memory (TRM) CD8+ T lymphocytes, myeloid-derived suppressor cells (MDSCs), and alternatively activated M2 macrophages. Importantly, prophylactic metformin treatment reversed these characteristics including aberrant p21WAF1/CIP1 expression and subsequently reduced HCC incidence in Ncoa5+/- male mice. Heterozygous deletion of the p21WAF1/CIP1 gene alleviated the key features associated with the protumorigenic niche in the livers of Ncoa5+/- male mice. Moreover, transcriptomic analysis reveals that preneoplastic livers of Ncoa5+/- mice are similar to the livers of nonalcoholic steatohepatitis patients as well as the adjacent noncancerous liver tissues of a subset of HCC patients with a relatively poor prognosis. Together, our results suggest that p21WAF1/CIP1 overexpression is essential in the development of protumorigenic microenvironment induced by NCOA5 deficiency and metformin prevents HCC development via alleviating p21WAF1/CIP1 overexpression and protumorigenic microenvironment.
中文翻译:

NCOA5缺乏症通过p21WAF1 / CIP1的过表达促进了独特的肝脏致瘤性微环境,而二甲双胍可以逆转这种过度。
肝细胞癌(HCC)的预防和治疗选择目前有限,强调了进一步阐明HCC发生的分子机制并确定新的预防和治疗靶点的必要性。在这里,我们展示了肝癌的Ncoa5 +/-小鼠模型肝脏中独特的致瘤生境,其特征在于包括p21WAF1 / CIP1和促炎细胞因子基因在内的部分基因表达的改变,假定的肝祖细胞增加以及活化和扩增的肝癌的扩展。组织驻留记忆(TRM)CD8 + T淋巴细胞,骨髓来源的抑制细胞(MDSC),以及激活的M2巨噬细胞。重要的是,预防性二甲双胍治疗可逆转这些特征,包括异常的p21WAF1 / CIP1表达,并随后降低Ncoa5 +/-雄性小鼠的HCC发生率。p21WAF1 / CIP1基因的杂合缺失可减轻与Ncoa5 +/-雄性小鼠肝脏中成瘤性利基相关的关键特征。此外,转录组学分析显示,Ncoa5 +/-小鼠的肿瘤前肝与非酒精性脂肪性肝炎患者的肝以及部分预后相对较差的HCC患者的相邻非癌性肝组织相似。总之,我们的结果表明p21WAF1 / CIP1的过表达在NCOA5缺乏诱导的致癌微环境的发展中至关重要,二甲双胍通过减轻p21WAF1 / CIP1的过表达和致癌的微环境来预防HCC的发展。转录组分析显示,Ncoa5 +/-小鼠的肿瘤前肝与非酒精性脂肪性肝炎患者的肝以及部分预后相对较差的HCC患者的相邻非癌性肝组织相似。总之,我们的结果表明p21WAF1 / CIP1的过表达在NCOA5缺乏诱导的致癌微环境的发展中至关重要,二甲双胍通过减轻p21WAF1 / CIP1的过表达和致癌的微环境来预防HCC的发展。转录组分析显示,Ncoa5 +/-小鼠的肿瘤前肝与非酒精性脂肪性肝炎患者的肝以及部分预后相对较差的HCC患者的相邻非癌性肝组织相似。总之,我们的结果表明p21WAF1 / CIP1的过表达在NCOA5缺乏诱导的致癌微环境的发展中至关重要,二甲双胍通过减轻p21WAF1 / CIP1的过表达和致癌的微环境来预防HCC的发展。
更新日期:2020-03-20
中文翻译:

NCOA5缺乏症通过p21WAF1 / CIP1的过表达促进了独特的肝脏致瘤性微环境,而二甲双胍可以逆转这种过度。
肝细胞癌(HCC)的预防和治疗选择目前有限,强调了进一步阐明HCC发生的分子机制并确定新的预防和治疗靶点的必要性。在这里,我们展示了肝癌的Ncoa5 +/-小鼠模型肝脏中独特的致瘤生境,其特征在于包括p21WAF1 / CIP1和促炎细胞因子基因在内的部分基因表达的改变,假定的肝祖细胞增加以及活化和扩增的肝癌的扩展。组织驻留记忆(TRM)CD8 + T淋巴细胞,骨髓来源的抑制细胞(MDSC),以及激活的M2巨噬细胞。重要的是,预防性二甲双胍治疗可逆转这些特征,包括异常的p21WAF1 / CIP1表达,并随后降低Ncoa5 +/-雄性小鼠的HCC发生率。p21WAF1 / CIP1基因的杂合缺失可减轻与Ncoa5 +/-雄性小鼠肝脏中成瘤性利基相关的关键特征。此外,转录组学分析显示,Ncoa5 +/-小鼠的肿瘤前肝与非酒精性脂肪性肝炎患者的肝以及部分预后相对较差的HCC患者的相邻非癌性肝组织相似。总之,我们的结果表明p21WAF1 / CIP1的过表达在NCOA5缺乏诱导的致癌微环境的发展中至关重要,二甲双胍通过减轻p21WAF1 / CIP1的过表达和致癌的微环境来预防HCC的发展。转录组分析显示,Ncoa5 +/-小鼠的肿瘤前肝与非酒精性脂肪性肝炎患者的肝以及部分预后相对较差的HCC患者的相邻非癌性肝组织相似。总之,我们的结果表明p21WAF1 / CIP1的过表达在NCOA5缺乏诱导的致癌微环境的发展中至关重要,二甲双胍通过减轻p21WAF1 / CIP1的过表达和致癌的微环境来预防HCC的发展。转录组分析显示,Ncoa5 +/-小鼠的肿瘤前肝与非酒精性脂肪性肝炎患者的肝以及部分预后相对较差的HCC患者的相邻非癌性肝组织相似。总之,我们的结果表明p21WAF1 / CIP1的过表达在NCOA5缺乏诱导的致癌微环境的发展中至关重要,二甲双胍通过减轻p21WAF1 / CIP1的过表达和致癌的微环境来预防HCC的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号