当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different kinetics for the hepatic uptake of lipid nanoparticles between the apolipoprotein E/low density lipoprotein receptor and the N-acetyl-d-galactosamine/asialoglycoprotein receptor pathway.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.jconrel.2020.03.006 Yusuke Sato 1 , Yoshiyuki Kinami 1 , Kazuki Hashiba 1 , Hideyoshi Harashima 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.jconrel.2020.03.006 Yusuke Sato 1 , Yoshiyuki Kinami 1 , Kazuki Hashiba 1 , Hideyoshi Harashima 1
Affiliation
|
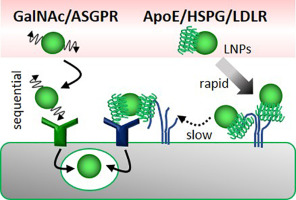
Lipid nanoparticles (LNPs) are one of the more promising technologies for efficiently delivering nucleic acids in vivo. Hepatocytes are the primary target cells of LNPs that are delivered via the apolipoprotein E (ApoE)-low density lipoprotein receptor (LDLR) pathway, an endogenous targeting pathway. This robust targeting mechanism results in the specific and efficient delivery of nucleic acids to hepatocytes. Trivalent N-acetyl-D-galactosamine (GalNAc) is known to be a high-affinity exogenous ligand against the asialoglycoprotein receptor (ASGPR), which is highly expressed on hepatocytes. In this study, we report that the kinetics of the hepatic uptake process between the two types of targeting pathways are different. Rapid blood clearance, accumulation to the space of Disse and a subsequent slow cellular uptake was observed in the case of the endogenous ApoE-LDLR pathway. On the other hand, both blood clearance and cellular uptake were more gradual in the case of the exogenous GalNAc-ASGPR pathway. Interactions between ApoE-bound LNPs and hepatic heparan sulfate proteoglycans (HSPGs) were involved in the rapid blood clearance and accumulation to the space of Disse in the case of the endogenous pathway. The findings presented here contribute to a more precise understanding of the mechanism of hepatic uptake and to the rational design of hepatocyte-targeting nanoparticles.
中文翻译:

载脂蛋白E /低密度脂蛋白受体与N-乙酰基-d-半乳糖胺/泛脂蛋白受体途径之间肝脂质纳米粒摄取的动力学不同。
脂质纳米颗粒(LNP)是在体内有效递送核酸的最有前途的技术之一。肝细胞是通过载脂蛋白E(ApoE)-低密度脂蛋白受体(LDLR)途径(内源性靶向途径)传递的LNP的主要靶细胞。这种强大的靶向机制导致了核酸向肝细胞的特异性和高效传递。已知三价N-乙酰基-D-半乳糖胺(GalNAc)是针对去唾液酸糖蛋白受体(ASGPR)的高亲和力外源性配体,该受体在肝细胞上高度表达。在这项研究中,我们报道了两种靶向途径之间肝吸收过程的动力学是不同的。快速血液清除 在内源性ApoE-LDLR通路的情况下,观察到Disse积累到Disse空间并随后缓慢吸收细胞。另一方面,在外源性GalNAc-ASGPR途径的情况下,血液清除和细胞摄取均较缓慢。在内源性途径的情况下,ApoE结合的LNP与肝素硫酸乙酰肝素蛋白聚糖(HSPG)之间的相互作用涉及血液的快速清除和在Disse空间的积累。此处提出的发现有助于更精确地了解肝吸收的机制,并有助于合理设计靶向肝细胞的纳米颗粒。在内源性途径的情况下,ApoE结合的LNP与肝素硫酸乙酰肝素蛋白聚糖(HSPG)之间的相互作用涉及血液的快速清除和在Disse空间的积累。此处提出的发现有助于更精确地了解肝吸收的机制,并有助于合理设计靶向肝细胞的纳米颗粒。在内源性途径的情况下,ApoE结合的LNP与肝素硫酸乙酰肝素蛋白聚糖(HSPG)之间的相互作用涉及血液的快速清除和在Disse空间的积累。此处提出的发现有助于更精确地了解肝吸收的机制,并有助于合理设计靶向肝细胞的纳米颗粒。
更新日期:2020-03-05
中文翻译:

载脂蛋白E /低密度脂蛋白受体与N-乙酰基-d-半乳糖胺/泛脂蛋白受体途径之间肝脂质纳米粒摄取的动力学不同。
脂质纳米颗粒(LNP)是在体内有效递送核酸的最有前途的技术之一。肝细胞是通过载脂蛋白E(ApoE)-低密度脂蛋白受体(LDLR)途径(内源性靶向途径)传递的LNP的主要靶细胞。这种强大的靶向机制导致了核酸向肝细胞的特异性和高效传递。已知三价N-乙酰基-D-半乳糖胺(GalNAc)是针对去唾液酸糖蛋白受体(ASGPR)的高亲和力外源性配体,该受体在肝细胞上高度表达。在这项研究中,我们报道了两种靶向途径之间肝吸收过程的动力学是不同的。快速血液清除 在内源性ApoE-LDLR通路的情况下,观察到Disse积累到Disse空间并随后缓慢吸收细胞。另一方面,在外源性GalNAc-ASGPR途径的情况下,血液清除和细胞摄取均较缓慢。在内源性途径的情况下,ApoE结合的LNP与肝素硫酸乙酰肝素蛋白聚糖(HSPG)之间的相互作用涉及血液的快速清除和在Disse空间的积累。此处提出的发现有助于更精确地了解肝吸收的机制,并有助于合理设计靶向肝细胞的纳米颗粒。在内源性途径的情况下,ApoE结合的LNP与肝素硫酸乙酰肝素蛋白聚糖(HSPG)之间的相互作用涉及血液的快速清除和在Disse空间的积累。此处提出的发现有助于更精确地了解肝吸收的机制,并有助于合理设计靶向肝细胞的纳米颗粒。在内源性途径的情况下,ApoE结合的LNP与肝素硫酸乙酰肝素蛋白聚糖(HSPG)之间的相互作用涉及血液的快速清除和在Disse空间的积累。此处提出的发现有助于更精确地了解肝吸收的机制,并有助于合理设计靶向肝细胞的纳米颗粒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号