当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing Surfaces in Dynamic Protein Interactions.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jmb.2020.02.032 Emil Spreitzer 1 , Sinem Usluer 1 , Tobias Madl 2
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jmb.2020.02.032 Emil Spreitzer 1 , Sinem Usluer 1 , Tobias Madl 2
Affiliation
|
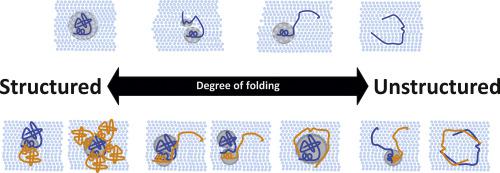
Proteins and their interactions control a plethora of biological functions and enable life. Protein-protein interactions can be highly dynamic, involve proteins with different degrees of "foldedness," and are often regulated through an intricate network of post-translational modifications. Central parts of protein-protein networks are intrinsically disordered proteins (IDPs). IDPs act as regulatory interaction hubs, enabled by their flexible nature. They employ various modes of binding mechanisms, from folding upon ligand binding to formation of highly dynamic "fuzzy" protein-protein complexes. Mutations or perturbations in regulation of IDPs are hallmarks of many diseases. Protein surfaces play key roles in protein-protein interactions. However, protein surfaces and protein surface accessibility are difficult to study experimentally. NMR-based solvent paramagnetic relaxation enhancement (sPRE) can provide quantitative experimental information on protein surface accessibility, which can be further used to obtain distance information for structure determination, identification of interaction surfaces, conformational changes, and identification of low-populated transient structure and long-range contacts in IDPs and dynamic protein-protein interactions. In this review, we present and discuss state-of the art sPRE techniques and their applications to investigate structure and dynamics of IDPs and protein-protein interactions. Finally, we provide an outline for potential future applications of the sPRE approach in combination with complementary techniques and modeling, to study novel paradigms, such as liquid-liquid phase separation, regulation of IDPs and protein-protein interactions by post-translational modifications, and targeting of disordered proteins.
中文翻译:

在动态蛋白质相互作用中探测表面。
蛋白质及其相互作用控制着过多的生物功能并使生命成为可能。蛋白质-蛋白质相互作用可以是高度动态的,涉及具有不同程度“折叠”的蛋白质,并且通常通过复杂的翻译后修饰网络进行调节。蛋白质-蛋白质网络的中心部分是本质上无序的蛋白质 (IDP)。IDP 因其灵活的性质而充当监管互动中心。它们采用各种模式的结合机制,从配体结合折叠到形成高度动态的“模糊”蛋白质-蛋白质复合物。IDPs 调控中的突变或扰动是许多疾病的标志。蛋白质表面在蛋白质-蛋白质相互作用中起关键作用。然而,蛋白质表面和蛋白质表面可及性难以通过实验研究。基于 NMR 的溶剂顺磁弛豫增强 (sPRE) 可以提供蛋白质表面可及性的定量实验信息,可进一步用于获取距离信息,用于结构确定、相互作用表面识别、构象变化以及低种群瞬态结构的识别和IDP 中的远程接触和动态蛋白质-蛋白质相互作用。在这篇综述中,我们介绍并讨论了最先进的 sPRE 技术及其在研究 IDP 的结构和动力学以及蛋白质-蛋白质相互作用中的应用。最后,我们为 sPRE 方法与互补技术和建模相结合的潜在未来应用提供了一个大纲,
更新日期:2020-03-02
中文翻译:

在动态蛋白质相互作用中探测表面。
蛋白质及其相互作用控制着过多的生物功能并使生命成为可能。蛋白质-蛋白质相互作用可以是高度动态的,涉及具有不同程度“折叠”的蛋白质,并且通常通过复杂的翻译后修饰网络进行调节。蛋白质-蛋白质网络的中心部分是本质上无序的蛋白质 (IDP)。IDP 因其灵活的性质而充当监管互动中心。它们采用各种模式的结合机制,从配体结合折叠到形成高度动态的“模糊”蛋白质-蛋白质复合物。IDPs 调控中的突变或扰动是许多疾病的标志。蛋白质表面在蛋白质-蛋白质相互作用中起关键作用。然而,蛋白质表面和蛋白质表面可及性难以通过实验研究。基于 NMR 的溶剂顺磁弛豫增强 (sPRE) 可以提供蛋白质表面可及性的定量实验信息,可进一步用于获取距离信息,用于结构确定、相互作用表面识别、构象变化以及低种群瞬态结构的识别和IDP 中的远程接触和动态蛋白质-蛋白质相互作用。在这篇综述中,我们介绍并讨论了最先进的 sPRE 技术及其在研究 IDP 的结构和动力学以及蛋白质-蛋白质相互作用中的应用。最后,我们为 sPRE 方法与互补技术和建模相结合的潜在未来应用提供了一个大纲,











































 京公网安备 11010802027423号
京公网安备 11010802027423号