当前位置:
X-MOL 学术
›
Nat. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-catalysed benzylic C-H coupling with alcohols via radical relay enabled by redox buffering.
Nature Catalysis ( IF 42.8 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41929-020-0425-1 Huayou Hu 1, 2, 3 , Si-Jie Chen 1, 2 , Mukunda Mandal 4 , Saied Md Pratik 4 , Joshua A Buss 1 , Shane W Krska 5 , Christopher J Cramer 4 , Shannon S Stahl 1
Nature Catalysis ( IF 42.8 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41929-020-0425-1 Huayou Hu 1, 2, 3 , Si-Jie Chen 1, 2 , Mukunda Mandal 4 , Saied Md Pratik 4 , Joshua A Buss 1 , Shane W Krska 5 , Christopher J Cramer 4 , Shannon S Stahl 1
Affiliation
|
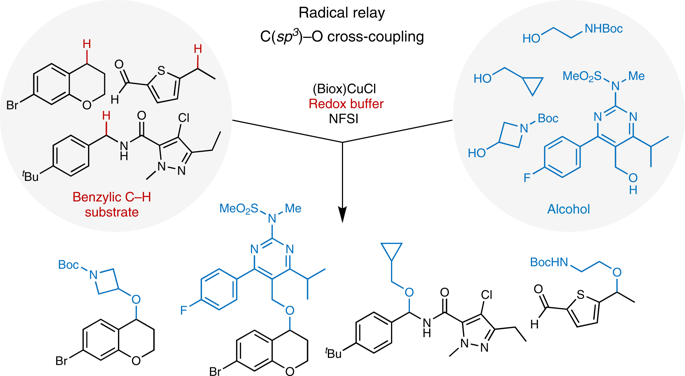
Cross-coupling reactions enable rapid, convergent synthesis of diverse molecules and provide the foundation for modern chemical synthesis. The most widely used methods employ sp2-hybridized coupling partners, such as aryl halides or related pre-functionalized substrates. Here, we demonstrate copper-catalysed oxidative cross coupling of benzylic C-H bonds with alcohols to afford benzyl ethers, enabled by a redox-buffering strategy that maintains the activity of the copper catalyst throughout the reaction. The reactions employ the C-H substrate as the limiting reagent and exhibit broad scope with respect to both coupling partners. This approach to direct site-selective functionalization of C(sp3)-H bonds provides the basis for efficient three-dimensional diversification of organic molecules and should find widespread utility in organic synthesis, particularly for medicinal chemistry applications.
中文翻译:

铜催化的苄基CH通过氧化还原缓冲的自由基中继与醇偶联。
交叉偶联反应可以快速,收敛地合成各种分子,并为现代化学合成提供了基础。最广泛使用的方法是使用sp2杂化的偶联伴侣,例如卤化芳基或相关的预功能化底物。在这里,我们展示了铜催化的苄基CH键与醇的氧化交叉偶联,以提供苄基醚,其通过氧化还原缓冲策略得以维持,该策略在整个反应过程中保持铜催化剂的活性。该反应使用CH底物作为限制试剂,并且相对于两种偶联配偶体均显示出广泛的范围。这种直接对C(sp3)-H键进行位点选择性官能化的方法为有机分子的有效三维多样化提供了基础,并且应该在有机合成中找到广泛的用途,
更新日期:2020-02-24
中文翻译:

铜催化的苄基CH通过氧化还原缓冲的自由基中继与醇偶联。
交叉偶联反应可以快速,收敛地合成各种分子,并为现代化学合成提供了基础。最广泛使用的方法是使用sp2杂化的偶联伴侣,例如卤化芳基或相关的预功能化底物。在这里,我们展示了铜催化的苄基CH键与醇的氧化交叉偶联,以提供苄基醚,其通过氧化还原缓冲策略得以维持,该策略在整个反应过程中保持铜催化剂的活性。该反应使用CH底物作为限制试剂,并且相对于两种偶联配偶体均显示出广泛的范围。这种直接对C(sp3)-H键进行位点选择性官能化的方法为有机分子的有效三维多样化提供了基础,并且应该在有机合成中找到广泛的用途,











































 京公网安备 11010802027423号
京公网安备 11010802027423号