当前位置:
X-MOL 学术
›
Nat. Rev. Nephrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Innate immunity in diabetic kidney disease.
Nature Reviews Nephrology ( IF 28.6 ) Pub Date : 2020-01-15 , DOI: 10.1038/s41581-019-0234-4 Sydney C W Tang 1 , Wai Han Yiu 1
Nature Reviews Nephrology ( IF 28.6 ) Pub Date : 2020-01-15 , DOI: 10.1038/s41581-019-0234-4 Sydney C W Tang 1 , Wai Han Yiu 1
Affiliation
|
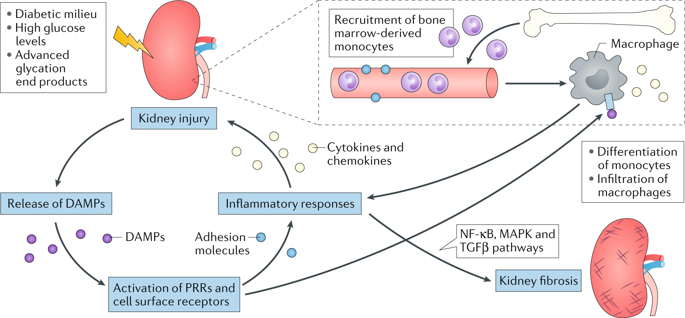
Increasing evidence suggests that renal inflammation contributes to the pathogenesis and progression of diabetic kidney disease (DKD) and that anti-inflammatory therapies might have renoprotective effects in DKD. Immune cells and resident renal cells that activate innate immunity have critical roles in triggering and sustaining inflammation in this setting. Evidence from clinical and experimental studies suggests that several innate immune pathways have potential roles in the pathogenesis and progression of DKD. Toll-like receptors detect endogenous danger-associated molecular patterns generated during diabetes and induce a sterile tubulointerstitial inflammatory response via the NF-κB signalling pathway. The NLRP3 inflammasome links sensing of metabolic stress in the diabetic kidney to activation of pro-inflammatory cascades via the induction of IL-1β and IL-18. The kallikrein-kinin system promotes inflammatory processes via the generation of bradykinins and the activation of bradykinin receptors, and activation of protease-activated receptors on kidney cells by coagulation enzymes contributes to renal inflammation and fibrosis in DKD. In addition, hyperglycaemia leads to protein glycation and activation of the complement cascade via recognition of glycated proteins by mannan-binding lectin and/or dysfunction of glycated complement regulatory proteins. Data from preclinical studies suggest that targeting these innate immune pathways could lead to novel therapies for DKD.
中文翻译:

糖尿病肾病的先天免疫。
越来越多的证据表明,肾脏炎症有助于糖尿病肾病 (DKD) 的发病机制和进展,并且抗炎疗法可能对 DKD 具有肾脏保护作用。在这种情况下,激活先天免疫的免疫细胞和常驻肾细胞在触发和维持炎症方面具有关键作用。来自临床和实验研究的证据表明,几种先天免疫途径在 DKD 的发病机制和进展中具有潜在作用。Toll 样受体检测糖尿病期间产生的内源性危险相关分子模式,并通过 NF-κB 信号通路诱导无菌小管间质炎症反应。NLRP3 炎性体通过诱导 IL-1β 和 IL-18 将糖尿病肾脏代谢应激的感知与促炎级联反应的激活联系起来。激肽释放酶-激肽系统通过产生缓激肽和激活缓激肽受体来促进炎症过程,而凝血酶激活肾细胞上的蛋白酶激活受体有助于 DKD 中的肾脏炎症和纤维化。此外,高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。激肽释放酶-激肽系统通过产生缓激肽和激活缓激肽受体来促进炎症过程,而凝血酶激活肾细胞上的蛋白酶激活受体有助于 DKD 中的肾脏炎症和纤维化。此外,高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。激肽释放酶-激肽系统通过产生缓激肽和激活缓激肽受体来促进炎症过程,而凝血酶激活肾细胞上的蛋白酶激活受体有助于 DKD 中的肾脏炎症和纤维化。此外,高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。
更新日期:2020-01-15
中文翻译:

糖尿病肾病的先天免疫。
越来越多的证据表明,肾脏炎症有助于糖尿病肾病 (DKD) 的发病机制和进展,并且抗炎疗法可能对 DKD 具有肾脏保护作用。在这种情况下,激活先天免疫的免疫细胞和常驻肾细胞在触发和维持炎症方面具有关键作用。来自临床和实验研究的证据表明,几种先天免疫途径在 DKD 的发病机制和进展中具有潜在作用。Toll 样受体检测糖尿病期间产生的内源性危险相关分子模式,并通过 NF-κB 信号通路诱导无菌小管间质炎症反应。NLRP3 炎性体通过诱导 IL-1β 和 IL-18 将糖尿病肾脏代谢应激的感知与促炎级联反应的激活联系起来。激肽释放酶-激肽系统通过产生缓激肽和激活缓激肽受体来促进炎症过程,而凝血酶激活肾细胞上的蛋白酶激活受体有助于 DKD 中的肾脏炎症和纤维化。此外,高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。激肽释放酶-激肽系统通过产生缓激肽和激活缓激肽受体来促进炎症过程,而凝血酶激活肾细胞上的蛋白酶激活受体有助于 DKD 中的肾脏炎症和纤维化。此外,高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。激肽释放酶-激肽系统通过产生缓激肽和激活缓激肽受体来促进炎症过程,而凝血酶激活肾细胞上的蛋白酶激活受体有助于 DKD 中的肾脏炎症和纤维化。此外,高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。高血糖通过甘露聚糖结合凝集素识别糖化蛋白质和/或糖化补体调节蛋白功能障碍导致蛋白质糖化和补体级联激活。来自临床前研究的数据表明,针对这些先天免疫途径可能会导致 DKD 的新疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号