Abstract
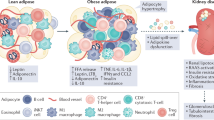
Increasing evidence suggests that renal inflammation contributes to the pathogenesis and progression of diabetic kidney disease (DKD) and that anti-inflammatory therapies might have renoprotective effects in DKD. Immune cells and resident renal cells that activate innate immunity have critical roles in triggering and sustaining inflammation in this setting. Evidence from clinical and experimental studies suggests that several innate immune pathways have potential roles in the pathogenesis and progression of DKD. Toll-like receptors detect endogenous danger-associated molecular patterns generated during diabetes and induce a sterile tubulointerstitial inflammatory response via the NF-κB signalling pathway. The NLRP3 inflammasome links sensing of metabolic stress in the diabetic kidney to activation of pro-inflammatory cascades via the induction of IL-1β and IL-18. The kallikrein–kinin system promotes inflammatory processes via the generation of bradykinins and the activation of bradykinin receptors, and activation of protease-activated receptors on kidney cells by coagulation enzymes contributes to renal inflammation and fibrosis in DKD. In addition, hyperglycaemia leads to protein glycation and activation of the complement cascade via recognition of glycated proteins by mannan-binding lectin and/or dysfunction of glycated complement regulatory proteins. Data from preclinical studies suggest that targeting these innate immune pathways could lead to novel therapies for DKD.
Key points
-
Renal inflammation involving the upregulation of inflammatory signalling pathways, release of cytokines and chemokines and infiltration of immune cells, contributes to the pathogenesis and progression of diabetic kidney disease (DKD).
-
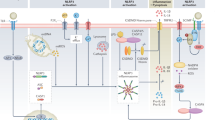
In the diabetic kidney, recognition of endogenous danger-associated molecular patterns by Toll-like receptors (TLRs), particularly TLR2 and TLR4, induces inflammatory responses.
-
Inflammasome activation not only amplifies renal inflammation but also has a role in the development of fibrosis; pharmacological agents that target inflammasome components may have therapeutic potential in DKD.
-
The kallikrein–kinin system and protease-activated receptor signalling have been implicated in the progression of DKD; inhibition of kallikrein using kallistatin is renoprotective in diabetic mice.
-
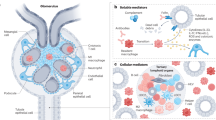
The complement system is activated in human DKD; C5a and C3a receptor antagonists improve kidney fibrosis in rats with DKD, supporting a pathogenetic role of complement in this disease.
-
Targeting inflammatory signalling pathways is a promising novel therapeutic approach for DKD; further studies of the roles of innate immunity pathways in DKD may lead to the identification of novel drug targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levey, A. S. & Coresh, J. Chronic kidney disease. Lancet 379, 165–180 (2012).
Ogurtsova, K. et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
Klessens, C. Q. F. et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 32, 1322–1329 (2017).
Nguyen, D. et al. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology 11, 226–231 (2006).
Moon, J. Y. et al. Aberrant recruitment and activation of T cells in diabetic nephropathy. Am. J. Nephrol. 35, 164–174 (2012).
Tang, S. C. et al. Bradykinin and high glucose promote renal tubular inflammation. Nephrol. Dial. Transplant. 25, 698–710 (2010).
Niewczas, M. A. et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 25, 805–813 (2019).
Woroniecka, K. I. et al. Transcriptome analysis of human diabetic kidney disease. Diabetes 60, 2354–2369 (2011).
Tang, S. et al. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J. Clin. Invest. 111, 515–527 (2003).
Tang, S. C. & Lai, K. N. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol. Dial. Transplant. 27, 3049–3056 (2012).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Chung, A. C. & Lan, H. Y. Chemokines in renal injury. J. Am. Soc. Nephrol. 22, 802–809 (2011).
Ferenbach, D., Kluth, D. C. & Hughes, J. Inflammatory cells in renal injury and repair. Semin. Nephrol. 27, 250–259 (2007).
Mack, M. & Yanagita, M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 87, 297–307 (2015).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10, 493–503 (2014).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799 (2019).
Perkins, B. A., Ficociello, L. H., Roshan, B., Warram, J. H. & Krolewski, A. S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 77, 57–64 (2010).
Saglimbene, V. et al. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J. Am. Soc. Nephrol. 29, 2890–2899 (2018).
Fried, L. F. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369, 1892–1903 (2013).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Moreno, J. A. et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin. Investig. Drugs 27, 917–930 (2018).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 6, 691–704 (2018).
Vallon, V. et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Ren. Physiol 306, F194–F204 (2014).
Panchapakesan, U. et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells-renoprotection in diabetic nephropathy? PLOS ONE 8, e54442 (2013).
Tang, L. et al. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 313, E563–E576 (2017).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Butler, J. et al. Empagliflozin improves kidney outcomes in patients with or without heart failure. Circ. Heart Fail 12, e005875 (2019).
Cherney, D. Z. I. et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 93, 231–244 (2018).
Chan, G. C. W. & Tang, S. C. W. SGLT2 inhibitor empagliflozin: finally at the latter stage of understanding? Kidney Int. 93, 22–24 (2018).
DeFronzo, R. A., Norton, L. & Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 13, 11–26 (2017).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Zavattaro, M. et al. One-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine 50, 620–626 (2015).
von Scholten, B. J., Hansen, T. W., Goetze, J. P., Persson, F. & Rossing, P. Glucagon-like peptide 1 receptor agonist (GLP-1 RA): long-term effect on kidney function in patients with type 2 diabetes. J. Diabetes Complications 29, 670–674 (2015).
Kodera, R. et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54, 965–978 (2011).
Park, C. W. et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 18, 1227–1238 (2007).
Balakumar, P., Kadian, S. & Mahadevan, N. Are PPAR alpha agonists a rational therapeutic strategy for preventing abnormalities of the diabetic kidney? Pharmacol. Res. 65, 430–436 (2012).
Kohan, D. E. & Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 86, 896–904 (2014).
Dhaun, N., Webb, D. J. & Kluth, D. C. Endothelin-1 and the kidney-beyond BP. Br. J. Pharmacol. 167, 720–731 (2012).
Anguiano, L., Riera, M., Pascual, J. & Soler, M. J. Endothelin blockade in diabetic kidney disease. J. Clin. Med. 4, 1171–1192 (2015).
Heerspink, H. J. L. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 393, 1937–1947 (2019).
Saleh, M. A., Boesen, E. I., Pollock, J. S., Savin, V. J. & Pollock, D. M. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56, 942–949 (2010).
Giunti, S., Barutta, F., Perin, P. C. & Gruden, G. Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr. Vasc. Pharmacol. 8, 849–860 (2010).
Kang, Y. S. et al. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 78, 883–894 (2010).
Seok, S. J. et al. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol. Dial. Transplant. 28, 1700–1710 (2013).
Sullivan, T. et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am. J. Physiol. Ren. Physiol. 305, F1288–F1297 (2013).
de Zeeuw, D. et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 3, 687–696 (2015).
Menne, J. et al. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dial. Transplant. 32, 307–315 (2017).
Gale, J. D. et al. Effect of PF-04634817, an oral CCR2/5 chemokine receptor antagonist, on albuminuria in adults with overt diabetic nephropathy. Kidney Int. Rep. 3, 1316–1327 (2018).
Tsalamandris, S. et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. 14, 50–59 (2019).
Jiang, T. et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59, 850–860 (2010).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
Chin, M. P. et al. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J. Card. Fail. 20, 953–958 (2014).
Chin, M. P. et al. Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes study. Am. J. Nephrol. 47, 40–47 (2018).
Nangaku, M., Shimazak, R. & Akizawa, T. Bardoxolone methyl improved GFR measured by standard inulin clearance: the TSUBAKI study [abstract SA-OR122]. J. Am. Soc. Nephrol. 28, B1 (2017).
Wong, C. K. et al. Aberrant expression of soluble co-stimulatory molecules and adhesion molecules in type 2 diabetic patients with nephropathy. J. Clin. immunol. 28, 36–43 (2008).
Li, H. Y. et al. Serum vascular adhesion protein-1 predicts end-stage renal disease in patients with type 2 diabetes. PLOS ONE 11, e0147981 (2016).
Salmi, M., Kalimo, K. & Jalkanen, S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J. Exp. Med. 178, 2255–2260 (1993).
Salmi, M. & Jalkanen, S. Vascular adhesion protein-1: a cell surface amine oxidase in translation. Antioxid. Redox Signal. 30, 314–332 (2019).
de Zeeuw, D. et al. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 6, 925–933 (2018).
Marrero, M. B., Banes-Berceli, A. K., Stern, D. M. & Eaton, D. C. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 290, F762–F768 (2006).
Berthier, C. C. et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58, 469–477 (2009).
Zhang, H. et al. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int. 92, 909–921 (2017).
Tuttle, K. R. et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant. 33, 1950–1959 (2018).
Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A. & Bazan, J. F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl Acad. Sci. USA 95, 588–593 (1998).
Takeda, K. & Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 (2005).
Lin, M. & Tang, S. C. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol. Dial. Transplant. 29, 746–754 (2014).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Moresco, E. M., LaVine, D. & Beutler, B. Toll-like receptors. Curr. Biol. 21, R488–R493 (2011).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Devaraj, S. et al. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 93, 578–583 (2008).
Devaraj, S., Dasu, M. R., Park, S. H. & Jialal, I. Increased levels of ligands of toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia 52, 1665–1668 (2009).
Dasu, M. R., Devaraj, S., Park, S. & Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33, 861–868 (2010).
Xu, X. H. et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104, 3103–3108 (2001).
Koc, M. et al. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol. Dial. Transplant. 26, 955–963 (2011).
Xu, Y. et al. Structural basis for signal transduction by the toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
De Nardo, D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine 74, 181–189 (2015).
Yiu, W. H., Lin, M. & Tang, S. C. Toll-like receptor activation: from renal inflammation to fibrosis. Kidney Int. Suppl. (2011) 4, 20–25 (2014).
Wu, H. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117, 2847–2859 (2007).
Pulskens, W. P. et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLOS ONE 3, e3596 (2008).
Chen, J. et al. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 79, 288–299 (2011).
Tang, S. C. et al. Additive renoprotective effects of B2-kinin receptor blocker and PPAR-γ agonist in uninephrectomized db/db mice. Lab. Invest. 91, 1351–1362 (2011).
Meldrum, K. K. et al. Profibrotic effect of interleukin-18 in HK-2 cells is dependent on stimulation of the toll-like receptor 4 (TLR4) promoter and increased TLR4 expression. J. Biol. Chem. 287, 40391–40399 (2012).
Lin, M. et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J. Am. Soc. Nephrol. 23, 86–102 (2012).
Verzola, D. et al. Enhanced glomerular Toll-like receptor 4 expression and signaling in patients with type 2 diabetic nephropathy and microalbuminuria. Kidney Int. 86, 1229–1243 (2014).
Ma, J. et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLOS ONE 9, e97985 (2014).
Jialal, I., Major, A. M. & Devaraj, S. Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J. Diabetes Complications 28, 755–761 (2014).
Ma, J. et al. Requirement for TLR2 in the development of albuminuria, inflammation and fibrosis in experimental diabetic nephropathy. Int. J. Clin. Exp. Pathol. 7, 481–495 (2014).
Wei, M., Li, Z., Xiao, L. & Yang, Z. Effects of ROS-relative NF-κB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol. Immunol. 68, 261–271 (2015).
Dasu, M. R., Devaraj, S., Zhao, L., Hwang, D. H. & Jialal, I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57, 3090–3098 (2008).
Mudaliar, H. et al. The role of toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am. J. Physiol. Ren. Physiol. 305, F143–F154 (2013).
Kaur, H., Chien, A. & Jialal, I. Hyperglycemia induces toll like receptor 4 expression and activity in mouse mesangial cells: relevance to diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 303, F1145–F1150 (2012).
Schaefer, L. et al. The matrix component biglycan is proinflammatory and signals through toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 115, 2223–2233 (2005).
Lewis, A. et al. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol. Histopathol. 23, 731–739 (2008).
Jheng, H. F. et al. Albumin stimulates renal tubular inflammation through an HSP70-TLR4 axis in mice with early diabetic nephropathy. Dis. Model. Mech. 8, 1311–1321 (2015).
Shi, H. et al. High mobility group box 1 in diabetic nephropathy. Exp. Ther. Med. 14, 2431–2433 (2017).
Chen, Q., Guan, X., Zuo, X., Wang, J. & Yin, W. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm. Sin. B 6, 183–188 (2016).
Chen, Y., Qiao, F., Zhao, Y., Wang, Y. & Liu, G. HMGB1 is activated in type 2 diabetes mellitus patients and in mesangial cells in response to high glucose. Int. J. Clin. Exp. Pathol. 8, 6683–6691 (2015).
Chen, B., Li, Y., Liu, Y. & Xu, Z. circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J. Cell Physiol. 234, 21249–21259 (2019).
Yao, D., Wang, S., Wang, M. & Lu, W. Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-κB signaling pathway. Mol. Med. Rep. 18, 3625–3630 (2018).
Jin, J. et al. Inhibition of high mobility group box 1 (HMGB1) attenuates podocyte apoptosis and epithelial-mesenchymal transition by regulating autophagy flux. J. Diabetes 11, 826–836 (2019).
Takahashi, T. & Harris, R. C. Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J. Diabetes Res. 2014, 590541 (2014).
Lin, M. et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 83, 887–900 (2013).
Cha, J. J. et al. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology 154, 2144–2155 (2013).
Liu, Z. M. et al. Low expression of miR-203 promoted diabetic nephropathy via increasing TLR4. Eur. Rev. Med. Pharmacol. Sci. 22, 5627–5634 (2018).
Ji, T.-T. et al. Long noncoding RNA Gm6135 functions as a competitive endogenous RNA to regulate toll-like receptor 4 expression by sponging miR-203-3p in diabetic nephropathy. J. Cell. Physiol. 234, 6633–6641 (2019).
Denby, L. & Baker, A. H. Targeting non-coding RNA for the therapy of renal disease. Curr. Opin. Pharmacol. 27, 70–77 (2016).
Caruso, R., Warner, N., Inohara, N. & Nunez, G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41, 898–908 (2014).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
Mulay, S. R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 96, 58–66 (2019).
Moossavi, M., Parsamanesh, N., Bahrami, A., Atkin, S. L. & Sahebkar, A. Role of the NLRP3 inflammasome in cancer. Mol. Cancer 17, 158 (2018).
Luan, J. & Ju, D. Inflammasome: a double-edged sword in liver diseases. Front. Immunol. 9, 2201 (2018).
Man, S. M. & Kanneganti, T. D. Regulation of inflammasome activation. Immunol. Rev. 265, 6–21 (2015).
Liu, Y., Xu, Z., Ma, F., Jia, Y. & Wang, G. Knockdown of TLR4 attenuates high glucose-induced podocyte injury via the NALP3/ASC/caspase-1 signaling pathway. Biomed. Pharmacother. 107, 1393–1401 (2018).
Fang, L. et al. Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PLOS ONE 8, e72344 (2013).
Shahzad, K. et al. Caspase-1, but not caspase-3, promotes diabetic nephropathy. J. Am. Soc. Nephrol. 27, 2270–2275 (2016).
Vilaysane, A. et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 21, 1732–1744 (2010).
Shahzad, K. et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 87, 74–84 (2015).
Gao, P. et al. Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim. Biophys. Acta 1843, 2448–2460 (2014).
Gao, P. et al. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J. Diabetes Res. 2015, 504761 (2015).
Fakhruddin, S., Alanazi, W. & Jackson, K. E. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J. Diabetes Res. 2017, 8379327 (2017).
Zhao, M. et al. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Dis. 4, 83–94 (2018).
Wu, M. et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell Endocrinol. 478, 115–125 (2018).
Tang, S. C., Yiu, W. H., Lin, M. & Lai, K. N. Diabetic nephropathy and proximal tubular damage. J. Ren. Nutr. 25, 230–233 (2015).
Gilbert, R. E. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 66, 791–800 (2017).
Song, S. et al. Knockdown of NLRP3 alleviates high glucose or TGFB1-induced EMT in human renal tubular cells. J. Mol. Endocrinol. 61, 101–113 (2018).
Qiao, Y. et al. Spleen tyrosine kinase promotes NLR family pyrin domain containing 3 inflammasome-mediated IL-1β secretion via c-Jun N-terminal kinase activation and cell apoptosis during diabetic nephropathy. Mol. Med. Rep. 18, 1995–2008 (2018).
Chen, K. et al. Optineurin inhibits NLRP3 inflammasome activation by enhancing mitophagy of renal tubular cells in diabetic nephropathy. FASEB J. 33, 4571–4585 (2019).
Wei, P. Z. & Szeto, C. C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta 496, 108–116 (2019).
Han, Y. et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 16, 32–46 (2018).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug. Discov. 17, 588–606 (2018).
Ozaki, E., Campbell, M. & Doyle, S. L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J. Inflamm. Res. 8, 15–27 (2015).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238 (2017).
Murphy, A. J. et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 23, 155–164 (2016).
Wang, W. et al. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J. Immunol. 190, 1239–1249 (2013).
Sharma, J. N. Role of tissue kallikrein-kininogen-kinin pathways in the cardiovascular system. Arch. Med. Res. 37, 299–306 (2006).
Chao, J. et al. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol. Chem. 391, 345–355 (2010).
Calixto, J. B. et al. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. Br. J. Pharmacol. 143, 803–818 (2004).
Chao, J. & Chao, L. Biochemistry, regulation and potential function of kallistatin. Biol. Chem. Hoppe Seyler 376, 705–713 (1995).
Ruggenenti, P., Cravedi, P. & Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 6, 319–330 (2010).
Kuoppala, A., Lindstedt, K. A., Saarinen, J., Kovanen, P. T. & Kokkonen, J. O. Inactivation of bradykinin by angiotensin-converting enzyme and by carboxypeptidase N in human plasma. Am. J. Physiol. Heart Circ. Physiol. 278, H1069–H1074 (2000).
Tschope, C. et al. Kinins are involved in the antiproteinuric effect of angiotensin-converting enzyme inhibition in experimental diabetic nephropathy. Int. Immunopharmacol. 3, 335–344 (2003).
Buleon, M. et al. Pharmacological blockade of B2-kinin receptor reduces renal protective effect of angiotensin-converting enzyme inhibition in db/db mice model. Am. J. Physiol. Ren. Physiol. 294, F1249–F1256 (2008).
Kwak, S. J. et al. Local kallikrein-kinin system is involved in podocyte apoptosis under diabetic conditions. Apoptosis 16, 478–490 (2011).
Bodin, S. et al. Kallikrein protects against microalbuminuria in experimental type I diabetes. Kidney Int. 76, 395–403 (2009).
Vitova, L. et al. Early urinary biomarkers of diabetic nephropathy in type 1 diabetes mellitus show involvement of kallikrein-kinin system. BMC Nephrol. 18, 112 (2017).
Campbell, D. J., Kelly, D. J., Wilkinson-Berka, J. L., Cooper, M. E. & Skinner, S. L. Increased bradykinin and “normal” angiotensin peptide levels in diabetic Sprague-Dawley and transgenic (mRen-2)27 rats. Kidney Int. 56, 211–221 (1999).
Campbell, D. J. et al. Increased tissue kallikrein levels in type 2 diabetes. Diabetologia 53, 779–785 (2010).
Tang, S. C., Leung, J. C. & Lai, K. N. The kallikrein-kinin system. Contrib. Nephrol. 170, 145–155 (2011).
Kakoki, M., McGarrah, R. W., Kim, H. S. & Smithies, O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc. Natl Acad. Sci. USA 104, 7576–7581 (2007).
Pereira, R. L. et al. Balance between the two kinin receptors in the progression of experimental focal and segmental glomerulosclerosis in mice. Dis. Model. Mech. 7, 701–710 (2014).
Mage, M. et al. Induction of B1 receptors in streptozotocin diabetic rats: possible involvement in the control of hyperglycemia-induced glomerular Erk 1 and 2 phosphorylation. Can. J. Physiol. Pharmacol. 80, 328–333 (2002).
Kakoki, M. et al. Lack of both bradykinin B1 and B2 receptors enhances nephropathy, neuropathy, and bone mineral loss in Akita diabetic mice. Proc. Natl Acad. Sci. USA 107, 10190–10195 (2010).
Liu, W. et al. Exogenous kallikrein protects against diabetic nephropathy. Kidney Int. 90, 1023–1036 (2016).
Yiu, W. H. et al. Tissue kallikrein mediates pro-inflammatory pathways and activation of protease-activated receptor-4 in proximal tubular epithelial cells. PLOS ONE 9, e88894 (2014).
Tan, Y., Wang, B., Keum, J. S. & Jaffa, A. A. Mechanisms through which bradykinin promotes glomerular injury in diabetes. Am. J. Physiol. Ren. Physiol. 288, F483–F492 (2005).
Qadri, F. & Bader, M. Kinin B1 receptors as a therapeutic target for inflammation. Expert. Opin. Ther. Targets 22, 31–44 (2018).
Ni, A., Chao, L. & Chao, J. Transcription factor nuclear factor kappaB regulates the inducible expression of the human B1 receptor gene in inflammation. J. Biol. Chem. 273, 2784–2791 (1998).
Liu, Y. et al. Depletion of endogenous kallistatin exacerbates renal and cardiovascular oxidative stress, inflammation, and organ remodeling. Am. J. Physiol. Ren. Physiol. 303, F1230–F1238 (2012).
Zhou, S. et al. Effects of kallistatin on oxidative stress and inflammation on renal ischemia-reperfusion injury in mice. Curr. Vasc. Pharmacol. 13, 265–273 (2015).
Li, P. et al. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology 142, 216–226 (2014).
Yiu, W. H. et al. Kallistatin protects against diabetic nephropathy in db/db mice by suppressing AGE-RAGE-induced oxidative stress. Kidney Int. 89, 386–398 (2016).
Adams, M. N. et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 130, 248–282 (2011).
Soh, U. J., Dores, M. R., Chen, B. & Trejo, J. Signal transduction by protease-activated receptors. Br. J. Pharmacol. 160, 191–203 (2010).
Mercer, P. F. & Chambers, R. C. Coagulation and coagulation signalling in fibrosis. Biochim. Biophys. Acta 1832, 1018–1027 (2013).
Isermann, B. Homeostatic effects of coagulation protease-dependent signaling and protease activated receptors. J. Thromb. Haemost. 15, 1273–1284 (2017).
Boire, A. et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120, 303–313 (2005).
Xu, Y. et al. Constitutive expression and modulation of the functional thrombin receptor in the human kidney. Am. J. Pathol. 146, 101–110 (1995).
Grandaliano, G. et al. Regenerative and proinflammatory effects of thrombin on human proximal tubular cells. J. Am. Soc. Nephrol. 11, 1016–1025 (2000).
Vesey, D. A., Hooper, J. D., Gobe, G. C. & Johnson, D. W. Potential physiological and pathophysiological roles for protease-activated receptor-2 in the kidney. Nephrology 12, 36–43 (2007).
Gui, Y., Loutzenhiser, R. & Hollenberg, M. D. Bidirectional regulation of renal hemodynamics by activation of PAR1 and PAR2 in isolated perfused rat kidney. Am. J. Physiol. Ren. Physiol. 285, F95–F104 (2003).
Hocherl, K., Gerl, M. & Schweda, F. Proteinase-activated receptors 1 and 2 exert opposite effects on renal renin release. Hypertension 58, 611–618 (2011).
Madhusudhan, T. et al. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood 119, 874–883 (2012).
Palygin, O., Ilatovskaya, D. V. & Staruschenko, A. Protease-activated receptors in kidney disease progression. Am. J. Physiol. Ren. Physiol. 311, F1140–F1144 (2016).
Jansen, M. P. B., Florquin, S. & Roelofs, J. The role of platelets in acute kidney injury. Nat. Rev. Nephrol. 14, 457–471 (2018).
Waasdorp, M., Duitman, J., Florquin, S. & Spek, C. A. Protease-activated receptor-1 deficiency protects against streptozotocin-induced diabetic nephropathy in mice. Sci. Rep. 6, 33030 (2016).
Cunningham, M. A. et al. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J. Exp. Med. 191, 455–462 (2000).
Grandaliano, G. et al. Protease-activated receptor-2 expression in IgA nephropathy: a potential role in the pathogenesis of interstitial fibrosis. J. Am. Soc. Nephrol. 14, 2072–2083 (2003).
Wang, Y. et al. Role of protease-activated receptor 2 in regulating focal segmental glomerulosclerosis. Cell Physiol. Biochem. 41, 1147–1155 (2017).
Du, C. et al. Protease-activated receptor-2 promotes kidney tubular epithelial inflammation by inhibiting autophagy via the PI3K/Akt/mTOR signalling pathway. Biochem. J. 474, 2733–2747 (2017).
Huang, M. J. et al. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open 7, e014294 (2017).
Pan, L. et al. Clinical significance of hemostatic parameters in the prediction for type 2 diabetes mellitus and diabetic nephropathy. Dis. Markers 2018, 5214376 (2018).
Sun, J. & Liu, C. Correlation of vascular endothelial function and coagulation factors with renal function and inflammatory factors in patients with diabetic nephropathy. Exp. Ther. Med. 16, 4167–4171 (2018).
Sumi, A. et al. Roles of coagulation pathway and factor Xa in the progression of diabetic nephropathy in db/db mice. Biol. Pharm. Bull. 34, 824–830 (2011).
Sakai, T. et al. Up-regulation of protease-activated receptor-1 in diabetic glomerulosclerosis. Biochem. Biophys. Res. Commun. 384, 173–179 (2009).
Kaizuka, M., Yamabe, H., Osawa, H., Okumura, K. & Fujimoto, N. Thrombin stimulates synthesis of type IV collagen and tissue inhibitor of metalloproteinases-1 by cultured human mesangial cells. J. Am. Soc. Nephrol. 10, 1516–1523 (1999).
Sharma, R. et al. Thrombin-induced podocyte injury is protease-activated receptor dependent. J. Am. Soc. Nephrol. 28, 2618–2630 (2017).
Wang, H. et al. Low but sustained coagulation activation ameliorates glucose-induced podocyte apoptosis: protective effect of factor V Leiden in diabetic nephropathy. Blood 117, 5231–5242 (2011).
Cohen, A. T. et al. Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLOS ONE 10, e0144856 (2015).
Feldberg, J. et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol. Dial. Transplant. 34, 265–277 (2019).
Oe, Y. et al. Coagulation factor Xa and protease-activated receptor 2 as novel therapeutic targets for diabetic nephropathy. Arterioscler. Thromb. Vasc. Biol. 36, 1525–1533 (2016).
Waasdorp, M., Duitman, J., Florquin, S. & Spek, A. C. Protease activated receptor 2 in diabetic nephropathy: a double edged sword. Am. J. Transl. Res. 9, 4512–4520 (2017).
Arif, S. A., D’Souza, J., Gil, M. & Gim, S. Vorapaxar for reduction of thrombotic cardiovascular events in myocardial infarction and peripheral artery disease. Am. J. Health Syst. Pharm. 72, 1615–1622 (2015).
Waasdorp, M., Duitman, J., Florquin, S. & Spek, C. A. Vorapaxar treatment reduces mesangial expansion in streptozotocin-induced diabetic nephropathy in mice. Oncotarget 9, 21655–21662 (2018).
Waasdorp, M., Florquin, S., Duitman, J. & Spek, C. A. Pharmacological PAR-1 inhibition reduces blood glucose levels but does not improve kidney function in experimental type 2 diabetic nephropathy. FASEB J. 33, 10966–10972 (2019).
Ungar, L. et al. Stroke outcomes with vorapaxar versus placebo in patients with acute coronary syndromes: insights from the TRACER trial. J. Am. Heart Assoc. 7, e009609 (2018).
Hajishengallis, G., Reis, E. S., Mastellos, D. C., Ricklin, D. & Lambris, J. D. Novel mechanisms and functions of complement. Nat. Immunol. 18, 1288–1298 (2017).
Hansen, S. et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J. Immunol. 185, 6096–6104 (2010).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Harris, C. L., Pouw, R. B., Kavanagh, D., Sun, R. & Ricklin, D. Developments in anti-complement therapy; from disease to clinical trial. Mol. Immunol. 102, 89–119 (2018).
Hillmen, P. et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 355, 1233–1243 (2006).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Tang, S., Zhou, W., Sheerin, N. S., Vaughan, R. W. & Sacks, S. H. Contribution of renal secreted complement C3 to the circulating pool in humans. J. Immunol. 162, 4336–4341 (1999).
Biancone, L. et al. Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int. 45, 451–460 (1994).
Ricklin, D., Mastellos, D. C., Reis, E. S. & Lambris, J. D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14, 26–47 (2018).
Wong, E. K., Goodship, T. H. & Kavanagh, D. Complement therapy in atypical haemolytic uraemic syndrome (aHUS). Mol. Immunol. 56, 199–212 (2013).
Smith, R. J. H. et al. C3 glomerulopathy – understanding a rare complement-driven renal disease. Nat. Rev. Nephrol. 15, 129–143 (2019).
Zipfel, P. F. et al. Complement inhibitors in clinical trials for glomerular diseases. Front. Immunol. 10, 2166 (2019).
Tatapudi, V. S. & Montgomery, R. A. Therapeutic modulation of the complement system in kidney transplantation: clinical indications and emerging drug leads. Front. Immunol. 10, 2306 (2019).
Biglarnia, A. R., Huber-Lang, M., Mohlin, C., Ekdahl, K. N. & Nilsson, B. The multifaceted role of complement in kidney transplantation. Nat. Rev. Nephrol. 14, 767–781 (2018).
Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 13, 311–318 (2017).
Yiu, W. H. et al. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol. Dial. Transplant. 33, 1323–1332 (2018).
Sircar, M. et al. Complement 7 is up-regulated in human early diabetic kidney disease. Am. J. Pathol. 188, 2147–2154 (2018).
Sun, Z. J. et al. Complement deposition on renal histopathology of patients with diabetic nephropathy. Diabetes Metab. 45, 363–368 (2019).
Vaisar, T. et al. Urine complement proteins and the risk of kidney disease progression and mortality in type 2 diabetes. Diabetes Care 41, 2361–2369 (2018).
Zheng, J. M. et al. Pathological significance of urinary complement activation in diabetic nephropathy: a full view from the development of the disease. J. Diabetes Investig. 10, 738–744 (2019).
Fujita, T. et al. Complement activation accelerates glomerular injury in diabetic rats. Nephron 81, 208–214 (1999).
Wang, H. et al. The lectin-like domain of thrombomodulin ameliorates diabetic glomerulopathy via complement inhibition. Thromb. Haemost. 108, 1141–1153 (2012).
Li, L. et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism 64, 597–610 (2015).
Li, L. et al. C3a receptor antagonist ameliorates inflammatory and fibrotic signals in type 2 diabetic nephropathy by suppressing the activation of TGF-β/smad3 and IKBα pathway. PLOS ONE 9, e113639 (2014).
Muller, L. M. et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 41, 281–288 (2005).
Kato, S. et al. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 3, 1526–1533 (2008).
Acknowledgements
The work of the authors is supported by the Research Grants Council (RGC, grant nos. C7018-16G, 17119818, 17151716), the Health and Medical Research Fund (HMRF, grant no. 05163596) of Hong Kong, the National Natural Science Fund (NSFC, grant no. 81870496) of China and by philanthropic donations from Winston Leung, K. K. Chan, Rita Liu (L & T Charitable Foundation Ltd.) and an Endowment Fund established at the University of Hong Kong for the Yu Professorship in Nephrology awarded to S.C.W.T.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks Jesus Egido, Jun Wada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Sterile inflammation
-
Pathogen-free inflammation triggered by damage-associated molecular patterns that are released by cells in response to stress.
- Homeostasis model assessment–insulin resistance
-
HOMA-IR. A method for evaluation of insulin sensitivity from basal (fasting) blood glucose and insulin levels.
- Factor V Leiden (FVL) mutation
-
A genetic point mutation (R506Q) in the gene that encodes human coagulation factor V that results in resistance of factor V to inactivation by activated protein C and an increase in blood clotting. Carriers of the FVL mutation have an increased risk of venous thrombosis.
- Lectins
-
Pattern recognition molecules that contain a C-type lectin domain (also known as a carbohydrate recognition domain).
- Ingenuity Pathway Analysis
-
IPA. A web-based software application for analysis, integration and interpretation of data from high-throughput experiments such as next generation sequencing and microarray. IPA aids in the identification of key regulators and activities of biological systems.
Rights and permissions
About this article
Cite this article
Tang, S.C.W., Yiu, W.H. Innate immunity in diabetic kidney disease. Nat Rev Nephrol 16, 206–222 (2020). https://doi.org/10.1038/s41581-019-0234-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0234-4
This article is cited by
-
Large-scale causal analysis of gut microbiota and six common complications of diabetes: a mendelian randomization study
Diabetology & Metabolic Syndrome (2024)
-
(Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway
Journal of Translational Medicine (2024)
-
Acetyl-CoA synthetase 2 promotes diabetic renal tubular injury in mice by rewiring fatty acid metabolism through SIRT1/ChREBP pathway
Acta Pharmacologica Sinica (2024)
-
Single-Cell Sequencing Reveals the Expression of Immune-Related Genes in Macrophages of Diabetic Kidney Disease
Inflammation (2024)
-
HMGN1 down-regulation in the diabetic kidney attenuates tubular cells injury and protects against renal inflammation via suppressing MCP-1 and KIM-1 expression through TLR4
Journal of Endocrinological Investigation (2024)