Laboratory Investigation ( IF 5.1 ) Pub Date : 2020-01-02 , DOI: 10.1038/s41374-019-0367-x Jie Zhang 1, 2 , Liang Wang 1, 2 , Jun Chu 1, 2 , Xiang Ao 1, 2 , Tao Jiang 1, 2 , Bin Yan 1, 2 , Minjun Huang 1, 2 , Zhongmin Zhang 1, 2
|
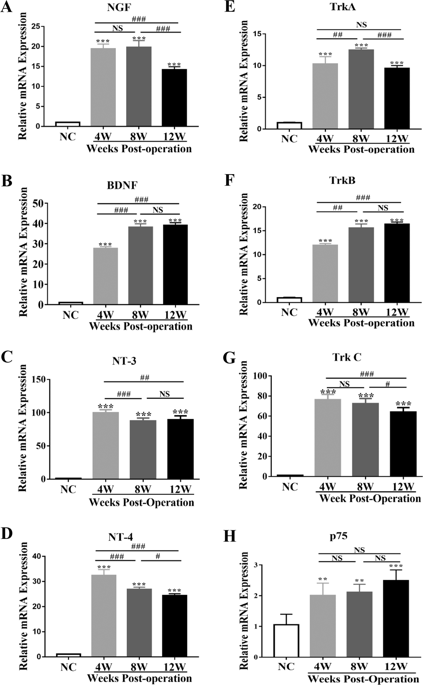
Heterotopic ossification (HO) is a debilitating condition that results from traumatic injuries or genetic diseases, for which the underlying mechanisms remain unclear. Recently, we have demonstrated the expression of neurotrophin-3 (NT-3) and its role in promoting HO formation via mediating endothelial–mesenchymal transition (EndMT) of vascular endothelial cells. The current study investigated the role of NT-3 on the surrounding mesenchymal cells and its potential origin throughout HO formation at injured Achilles tendons in rats. We used an Achilles tenotomy to induce HO formation in vivo and cultured primary tendon-derived stem cells (TDSCs) to investigate the underlying mechanisms mediating the osteogenesis in vitro. Furthermore, RAW264.7 cells were employed to identify the origin of NT-3. The mRNA levels of NGF, BDNF, NT-3, and NT-4 and their tyrosine protein kinase (Trk) receptors as well as p75 receptor were elevated at injury sites. NT-3 and TrkC showed the highest induction. Neutralization of the NT-3-induced effects by the pan-Trk inhibitor GNF5837 reduced the expression of bone/cartilage-related genes while injection of NT-3 promoted HO formation with elevated mRNA of bone/cartilage-related markers at injured sites. In vitro, NT-3 accelerated osteogenic differentiation and mineralization of TDSCs through activation of the ERK1/2 and PI3K/Akt signaling pathways. Moreover, the colocalization of NT-3 and macrophages, including M1 and M2 macrophages, was observed in injured sites throughout HO formation, and in vitro studies demonstrated that activated macrophages mediated the secretion of NT-3. In addition, an increasing concentration of serum or supernatant NT-3 was observed both in vivo and in vitro. Depletion of macrophages with clodronate-loaded liposomes reduced HO formation as well as secretion and mRNA expression of NT-3. Our study suggests that macrophage-derived NT-3 may promote HO formation and osteogenesis of TDSCs via the ERK1/2 and PI3K/Akt signaling pathways, which may provide new insights for the therapeutic directions of HO in the future.
中文翻译:

巨噬细胞衍生的神经营养蛋白 3 促进大鼠异位骨化
异位骨化 (HO) 是一种由外伤或遗传疾病引起的衰弱性疾病,其潜在机制尚不清楚。最近,我们已经证明了神经营养因子 3 (NT-3) 的表达及其在通过介导血管内皮细胞的内皮-间质转化 (EndMT) 促进 HO 形成中的作用。目前的研究调查了 NT-3 对周围间充质细胞的作用及其在大鼠跟腱损伤整个 HO 形成过程中的潜在起源。我们使用跟腱切开术在体内诱导 H2O 形成,并培养原代肌腱衍生干细胞 (TDSC) 以研究介导体外成骨的潜在机制。此外,RAW264.7 细胞用于鉴定 NT-3 的来源。NGF、BDNF、NT-3、和 NT-4 及其酪氨酸蛋白激酶 (Trk) 受体以及 p75 受体在损伤部位升高。NT-3 和 TrkC 表现出最高的诱导。pan-Trk 抑制剂 GNF5837 对 NT-3 诱导作用的中和降低了骨/软骨相关基因的表达,而注射 NT-3 促进了 HO 形成,在受伤部位骨/软骨相关标记物的 mRNA 升高。在体外,NT-3 通过激活 ERK1/2 和 PI3K/Akt 信号通路加速 TDSC 的成骨分化和矿化。此外,NT-3 和巨噬细胞(包括 M1 和 M2 巨噬细胞)在 HO 形成过程中的受损部位共定位,体外研究表明活化的巨噬细胞介导了 NT-3 的分泌。此外,在体内和体外均观察到血清或上清液 NT-3 浓度增加。用载有氯膦酸盐的脂质体去除巨噬细胞会减少 H2O 的形成以及 NT-3 的分泌和 mRNA 表达。我们的研究表明,巨噬细胞来源的 NT-3 可能通过 ERK1/2 和 PI3K/Akt 信号通路促进 TDSCs 的 HO 形成和成骨,这可能为未来 HO 的治疗方向提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号