Abstract
Heterotopic ossification (HO) is a debilitating condition that results from traumatic injuries or genetic diseases, for which the underlying mechanisms remain unclear. Recently, we have demonstrated the expression of neurotrophin-3 (NT-3) and its role in promoting HO formation via mediating endothelial–mesenchymal transition (EndMT) of vascular endothelial cells. The current study investigated the role of NT-3 on the surrounding mesenchymal cells and its potential origin throughout HO formation at injured Achilles tendons in rats. We used an Achilles tenotomy to induce HO formation in vivo and cultured primary tendon-derived stem cells (TDSCs) to investigate the underlying mechanisms mediating the osteogenesis in vitro. Furthermore, RAW264.7 cells were employed to identify the origin of NT-3. The mRNA levels of NGF, BDNF, NT-3, and NT-4 and their tyrosine protein kinase (Trk) receptors as well as p75 receptor were elevated at injury sites. NT-3 and TrkC showed the highest induction. Neutralization of the NT-3-induced effects by the pan-Trk inhibitor GNF5837 reduced the expression of bone/cartilage-related genes while injection of NT-3 promoted HO formation with elevated mRNA of bone/cartilage-related markers at injured sites. In vitro, NT-3 accelerated osteogenic differentiation and mineralization of TDSCs through activation of the ERK1/2 and PI3K/Akt signaling pathways. Moreover, the colocalization of NT-3 and macrophages, including M1 and M2 macrophages, was observed in injured sites throughout HO formation, and in vitro studies demonstrated that activated macrophages mediated the secretion of NT-3. In addition, an increasing concentration of serum or supernatant NT-3 was observed both in vivo and in vitro. Depletion of macrophages with clodronate-loaded liposomes reduced HO formation as well as secretion and mRNA expression of NT-3. Our study suggests that macrophage-derived NT-3 may promote HO formation and osteogenesis of TDSCs via the ERK1/2 and PI3K/Akt signaling pathways, which may provide new insights for the therapeutic directions of HO in the future.
Similar content being viewed by others
Introduction
Heterotopic ossification (HO) is characterized as the abnormal formation of ectopic bone in extraskeletal soft tissues [1]. HO is primarily acquired as a complication of major connective tissue injuries, traumatic central nervous system injuries, and surgical interventions. In rare instances, a particularly devastating form called fibrodysplasia ossificans progressiva is observed [2, 3]. HO can cause significant pain and gradual reduction in the range of motion of affected limbs, finally leading to disability. Despite the high reoccurrence rate, the only effective therapy for HO is surgical resection, which is a challenging procedure, particularly when ectopic bone entraps large blood vessels and nerves [4]. Therefore, a better understanding of the pathogenesis of HO is required for improving therapeutic strategies.
Progression of HO may be correlated with inappropriate differentiation of potential osteoprogenitor stem cells into osteoblasts under the influence of various cytokines [5]. In addition to transforming growth factor-beta superfamily members, including bone morphogenetic proteins (BMPs) and other inflammatory factors [6], a unique family of polypeptide growth factors called neurotrophins (NTs) participates in the regulation of proliferation, survival, migration, and differentiation of cells in the nervous system [7]. To date, four NTs have been identified and isolated: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), along with their receptors, including the high-affinity tropomyosin-related kinase (Trk) receptor tyrosine kinases (with NGF binding to TrkA; BDNF and NT-4 binding to TrkB; and NT-3 binding to TrkC) and the low affinity p75 neurotrophin receptor (p75, which can bind to all NTs) [8]. Currently, in addition to its effects in the nervous system, NT-3 has been shown to play a role in the skeletal system [9]. NT-3 was observed in the injured tibial growth plate and bone and promoted osteogenesis, vascularization and bony repair at the injured site [10, 11]. Similarly, in rat models of femoral distraction or rib fracture healing, NT-3 localization was observed in chondrocytes and osteoblasts during the endochondral ossification process [12, 13]. In addition, NT-3 was shown to improve fracture healing in rats by regulating the tibia BMD, biomechanical indexes, and bone formation [14]. Moreover, in a recent study, NT-3 was observed to be notably induced at the injured Achilles tendons and found to promote ectopic bone formation [15]. Despite these studies, it remains largely unclear on the origin of injury site-derived NT-3 and how NT-3 is involved in regulating the pathogenesis of HO formation.
HO is believed to develop through a process of endochondral ossification in four stages: inflammation, chondrogenesis, osteogenesis, and maturation [16]. In the inflammation stage of HO, immune cells such as monocytes and macrophages infiltrate the injured sites, causing the recruitment of progenitor cells and the secretion of cytokines. As a regulator of the inflammatory response, macrophages, which can control inflammation, have a role in ectopic bone formation [17]. A recent study described the triggering effect of macrophage-mediated inflammation in a spinal cord injury (SCI)-induced neurogenic HO mouse model [18]. In addition, a previous study reported that activated macrophages contributed to neurogenic HO formation by secreting oncostatin M to muscle cells within an inflammatory environment [19]. Interestingly, according to another SCI rat study, NT-3 was demonstrated to have a neuroprotective effect and activate macrophages in an inflammatory context [20]. Furthermore, a previous study suggested that macrophages could constitutively express NGF and BDNF in human studies and animal models [21]. However, the potential relationship between NT-3 and macrophages in the inflammatory environment of HO remains unclear. Therefore, in our current study, the surgically injured Achilles tendons of rats was employed to explore the underlying the mechanisms of NT-3 in HO formation, as well as the origin of injury site-derived NT-3 in the inflammatory situation.
Materials and methods
Rat Achilles tenotomy model and treatment
All animal procedures were approved by the Ethical Committee for Animal Research of Southern Medical University. All experiments were performed on 6-week-old Sprague Dawley (SD) rats (regardless of gender) obtained from the Laboratory Animal Center of Southern Medical University. One hundred and eight rats were anesthetized with a mixture of ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Then, 90 rats underwent a bilateral midpoint Achilles tenotomy through a posterior approach under aseptic conditions [16]. The incision was routinely closed with an interrupted 4–0 silk suture. Normal control rats (n = 6) were anaesthetized and underwent sham operations. All rats were left to recover on a heat pad at 37 °C until they were awake and returned to their cages.
For gene expression studies and histological analyses, 24 rats were euthanized at 4, 8, and 12 weeks post operation (n = 6/time point); the normal control group was also euthanized (n = 6). The Achilles tendons of the left limb acquired from all groups were collected and stored at −80 °C for relative gene expression analyses. For histological studies, all right limb specimens were fixed in 4% paraformaldehyde for 24 h, decalcified in 14% EDTA (pH 7.2–7.4), and processed for paraffin embedding to collect 4-μm-thick sections.
A challenge in studying the NT-3-related pathway is the lack of receptor-specific inhibitors as molecular reagents, here we employed the small pan-Trk inhibitor molecule GNF5837 to study the function of NT-3-TrkC signaling in vivo and in vitro [22]. For the functional study, 72 injured rats were randomly divided into four groups: the HO group, the rhNT-3 group, the pan-Trk inhibitor (GNF5837) group, and the saline group (n = 18/group). Rats from the rhNT-3 group received a weekly injection of rhNT-3 (1.2 mg/ml, 12 mg injection per week) surrounding the injured Achilles tendons. In the GNF5837 group, rats received an injection of the pan-Trk inhibitor GNF5837 (1 mg/kg) (Tocris Bioscience, Bristol, UK) once a week at the injured Achilles tendons. Rats in the saline group were administered a saline vehicle weekly, and rats in the HO group were used as a positive control. At 4, 8, and 12 weeks post operation, all rats were euthanized, and their limbs were collected as mentioned above for histological analyses, relative gene expression studies and micro-CT scanning (n = 6/group/time point).
For macrophage depletion study, another 12 injured rats were injected intravenously with liposomes containing clodronate (5 ul/g) [18]. The injection was performed immediately after Achilles tenotomy and every 3 days for 12 weeks after surgery. Control group was injected with an equivalent volume of PBS-loaded liposomes under the same condition.
qRT-PCR gene expression analyses
Total RNA was extracted with TRIzol reagent (Life Technologies, Grand Island, NY, USA) from Achilles tendon specimens, TDSCs or RAW264.7 macrophages at the indicated time points according to the manufacturer’s instructions. cDNA was synthesized with the TaKaRa PrimeScript RT Reagent Kit (TaKaRa Biotechnology Co., Ltd, Shija, Japan). A TaKaRa SYBR Premix Ex TaqII kit was used for qRT-PCR. The experiment was performed in triplicate, and the 2−△△CT method was used to analyze the relative gene expression levels. The corresponding primer sequences of the reference genes and the target genes are listed in Table 1.
Serum NT-3 level evaluation
Blood samples from experimental rats were collected and centrifuged at 2000 rpm for 10 min at 4 °C. Plasma fractions were collected and stored at −80 °C. The concentration of NT-3 in the serum was determined using the ELISA Kit for NT-3 (Rat: Cloud-Clone Corp. SEA106Ra) following the manufacturer’s instructions.
Immunohistochemistry (IHC) of NT-3 and TrkC
The time-course expression and localization of NT-3 and TrkC was assessed by IHC in paraffin sections using primary antibodies against NT-3 (1: 100, Abcam, ab65804) and TrkC (1:100, CST, 3376S). After incubation at 4 °C overnight, a species-matched HRP-labeled secondary antibody was used (1:500) at 37 °C for 1 h. For IHC, DAB (ZSGB-Bio, Beijing, China) was used as the chromogen, and hematoxylin was used for counterstaining.
Micro-CT analyses
Achilles tendons with calcaneus and lower tibias from rats in the experimental and control groups were fixed overnight in 4% paraformaldehyde and analyzed by micro-CT (µCT 80, Scanco Medical, Bruttisellen, Zurich, Switzerland). The scanner was set at a voltage of 60 kV at 150 μA and a resolution of 20 μm per pixel. Images were reconstructed for HO ectopic bone volume analyses.
Hematoxylin-eosin (H&E) and SOFG staining and image analyses
For visualization of ectopic bone and cartilage tissues within the injured Achilles tendon, hematoxylin-eosin (H&E) (Sigma–Aldrich, St. Louis, MI, USA) was performed. For histological analyses of HO, proportions of bone and bone marrow, as percentage of the total injury site areas, were quantified in the HO site of each slide on three contiguous sections from each rat of each group by ImageJ software.
IHC and immunofluorescence of macrophages
The time-course expression and localization of macrophages, as well as the coexpression of macrophages and NT-3, was assessed by IHC and immunofluorescence in paraffin sections using primary antibodies against CD68 (1:100, Abcam, ab31630), inducible nitric oxide synthase (iNOS) (1:100, Abcam, ab15323) and CD206 (1:500, Abcam, ab64693) for IHC; and NT-3 (1: 100, Abcam, ab65804), Stage-Specific Embryonic Antigen-4 (SSEA-4) (1:100, Abcam, ab16287), collagen I (1:100, Abcam, ab90395), CD68 (1:100, Abcam, ab955), iNOS (1:100, Abcam, ab15323) and CD206 (1:500, Abcam, ab64693) for immunofluorescence (IF). After incubation at 4 °C overnight, a species-matched Alexa Fluor 488-, Alexa Fluor 594- or HRP-labeled secondary antibody was used (1:500) at 37 °C for 1 hour. For IHC, DAB was used as mentioned above. For IF, sections were mounted with DAPI (Roche Applied Science, Indianapolis, IN, USA). For macrophages quantification, we counted the numbers of positively stained cells in five random visual fields in three sequential sections per rat in each group using ImagePro 4.5 software.
TDSC isolation, identification, and osteogenic differentiation
TDSCs were isolated from SD rats as described previously [23, 24]. Intact Achilles tendons without peritendinous tissues were obtained. Mid-substance tissues were minced and digested with collagenase I (Sigma–Aldrich) for 3 h at 37 °C. For suspended single-cell samples, the digested sample was passed through a 70 μm cell strainer (Becton Dickinson, Franklin Lakes, NJ). The released TDSCs were resuspended in growth medium containing Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Life Technologies, CA, USA), 100 mg/ml streptomycin, 100 U/ml penicillin, and 10% fetal bovine serum (Gibco, Grand Island, NY). The isolated TDSCs were incubated at 37 °C with 5% CO2 maintained to enable colony formation. Five days after initial plating, TDSCs were washed twice with DMEM to remove nonadherent cells. The medium was replaced every 3 days. Cells were then removed from the dish with 0.02% EDTA and 0.05% trypsin for passage. Third passage cells were used for further experiments. The adherent cells were identified as TDSCs by IF staining with SSEA-4 and collagen I (Supplementary Fig. B).
For osteogenic differentiation, TDSCs were seeded into six-well plates at a density of 1 × 105 cells/well in duplicate and cultured at 37 °C with 5% CO2. Twenty-four hours after seeding, the medium was removed and replaced with osteogenic medium (DMEM supplemented with 50 μmol/l ascorbic acid, 0.1 μmol/l dexamethasone, and 10 mmol/l β-glycerol phosphate) (Sigma–Aldrich). Cells were then incubated continuously in osteogenic medium for 21 days. For analysis of the osteogenic effects of NT-3, TDSCs were cultured in osteogenic medium with rhNT-3 (100 ng/ml) or the pan-Trk inhibitor GNF5837 (100 ng/ml) until the end day 21 with medium refreshed every 2–3 days, using normal osteogenic differentiation induction as a control.
To investigate the direct action of NT-3 on the activation of TrkC downstream kinases in TDSCs osteogenesis, TDSCs were seeded into six-well plates at a density of 1 × 105 cells/well in duplicate and cultured at 37 °C with 5% CO2 for 24 h. After that, TDSCs were serum-starved for 6 h and then cultured in osteogenic medium with rhNT-3 (100ng/ml), PD98059 (10uM) or LY294002(10 μM) for 2 days, using normal osteogenic differentiation induction as a control.
RAW264.7 macrophage culture and treatment
RAW264.7 macrophages (ATCC, MD, USA) were cultured in high-glucose DMEM with 10% fetal calf serum (Gibco, Life Technologies, CA, USA) in a 5% CO2 incubator at 37 °C. Every 48 h, the cells were subcultured by scraping. Cells were plated in six-well plates at a density of 1 × 106 cells/well and incubated under the same conditions overnight. Then, the cells were treated with either LPS (100 ng/ml), IL-6 (1 µg/ml), or IL-13 (1 µg/ml) for 0, 3, 6, 12, and 24 h. Total RNA and cell lysates were collected for qPCR and western blot analyses, cultured supernate was collected for ELISA assay.
Cell viability assay
TDSCs were cultured in 96-well plates at a density of 1 × 104 cells/well with various concentrations of rhNT-3 (1, 10, or 100 ng/ml) for 48 h. Cell viability was quantified using a Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay according to the manufacturer's instructions. The number of viable cells in each well was measured at an absorbance wavelength of 450 nm. (Supplementary Fig. C)
Alizarin Red staining
TDSCs were grown in six-well plates at a density of 1 × 105 cells/well with either rhNT-3, anti-NT-3, or normal control in osteogenic differentiation medium for 21 days. Alizarin Red staining (Sigma–Aldrich) was performed to determine the mineralization of TDSCs. Cells were washed with PBS, fixed with paraformaldehyde for 30 min, incubated with 1% Alizarin Red for 30 min, and washed with PBS to remove excess dye. After imaging, cells were solubilized with 1:1 acetic acid:methanol for 30 min, and the extracted stain was measured by spectrophotometry at 490 nm.
Western blot analysis
Cells lysates were collected and prepared for western blotting. We incubated primary antibodies against TrkC (1:1000 CST, 3376S), Runx2 (1:1000 CST, 12556S), OSX (1:1000 Abcam, ab22552), OCN (1:1000 Abcam, ab13420), ERK1/2 (1:1000 Abcam, ab17942), P-ERK1/2 (1:1000 CST, 9101S), PI3K (1:1000, Abcam ab151549), P-PI3K (1:1000, Abcam ab182651), Akt (1:1000, Abcam ab64148), P-Akt (1:1000, Abcam ab8933), P-signal transduction and activator of transcription 1 (P-STAT1) (1:1000 Abcam, ab30645), P-STAT3 (1:1000 Abcam, ab76315), P-STAT6 (1:1000 Abcam, ab28829), iNOS (1:1000 Abcam, ab15323), CD206 (1:1000, Abcam, ab64693) and GAPDH (ZSGB-Bio, China) overnight at 4 °C, followed by incubation with an anti-IgG horseradish peroxidase-conjugated secondary antibody (1:4000) for 1 h. Chemiluminescence was detected using an enhanced chemiluminescence system. GAPDH served as a loading control.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 (IBM, New York, NY, USA). All in vitro data are representative of three independent experiments, and each experiment was performed in triplicate. In vivo data represent n = 6 rats/group. Data are presented as the mean ± SD. The results were compared with one-way ANOVA for time-course studies without intervention treatments or two-way ANOVA for studies with different time points and intervention treatment groups, followed by the least significant difference post hoc test and Student’s t test. P < 0.05 was considered statistically significant.
Results
Expression of NTs and their receptors during HO formation at injured Achilles tendons
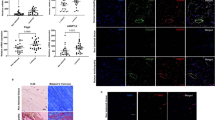
With regard to the NTs contributing to the formation of HO, there is still controversy. As the first step of this study, we investigated the mRNA levels of four NTs (NGF, BDNF, NT-3, and NT-4) and their receptors (TrkA, TrkB, TrkC as well as p75 receptor) at the rat Achilles tendons injured sites during HO formation, with the sham operation groups as normal controls. In comparison with normal controls, all NTs mRNA expression was increased from 4 weeks throughout 12 weeks post operation (p < 0.005–0.001). Among four NTs, when compared with normal controls, NT-3 expressed the highest induction (nearly 100-fold) while other NTs showed a modest induction (no more than 40-fold for BDNF and NT-4, and less than 20-fold for NGF) at the injured sites during HO formation (Fig. 1a–d). The mRNA expression of NT-3 and NT-4 was significantly elevated at 4 weeks then decreased from 8 weeks to 12 weeks. For NGF, mRNA levels were upregulated and maintained from 4 weeks to 8 weeks before a notably decrease at 12 weeks. For BDNF, there was a gradually rising in mRNA levels from 4 weeks to 12 weeks.
Time-course gene expression of NTs and their receptors at injured Achilles tendons. Expression levels (fold changes versus the normal control groups) of NGF (a), BDNF (b), NT-3 (c), and NT-4 (d) as well as receptors of TrkA (e), TrkB (f), TrkC (g), and p75 (h). (n = 6/group). All data represent the mean ± SD. *P < 0.05, **P < 0.005, and ***P < 0.001 versus the normal control. #P < 0.05, ##P < 0.005, and ###P < 0.001 versus each individual time point.
Similarly, in comparison with the controls, the expression of TrkC (nearly 80-fold, p < 0.001) showed a greater increase while TrkA (less than 15-fold, p < 0.001) and TrkB (less than 20-fold, p < 0.001) exhibited a little rising at the injured Achilles tendons during pathological process of HO (Fig. 1e, f, g). The mRNA expression trend of TrkB was consistent with BDNF, and TrkC was similar to NT-4. For TrkA, mRNA expression upregulated from 4 weeks to 8 weeks, following a droping at 12 weeks. Moreover, p75 receptor (less than threefold, p < 0.005) was obviously lower than that of high-affinity NTs receptors (Fig. 1h). These results suggest that NTs and the high-affinity Trk receptors were significantly induced during HO formation at injured site, particularly NT-3 and TrkC showing the highest expression. Based on these results, we believed that NT-3 might play a role in HO pathological process of injured Achilles tendons.
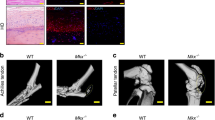
As a step to investigate mechanisms how NT-3 participates in HO formation, immunohistochemistry was conducted for visualization of NT-3 and TrkC during HO formation. According to our recent study [15], NT-3 and TrkC staining were mainly localized in infiltrating mesenchymal cells, osteoblasts, chondrocytes, vascular endothelial cells, and marrow cells from 4 weeks to 12 weeks during ectopic bone formation. Conversely, in the normal control groups, NT-3 and TrkC showed a low abundance in some mesenchymal cells. (Supplementary Fig. 1). These findings demonstrate that NT-3 and TrkC are significantly expressed during the HO formation phase at the injured Achilles tendon.
Effects of NT-3 on HO formation and expression of bone/cartilage-related genes at injured Achilles tendons
Previously, we have demonstrated the osteogenic effects of NT-3 on HO formation at injured Achilles tendons, and inhibition NT-3 suppressed HO formation [15]. Compared with other groups, rhNT-3 treatment showed a strong effect on HO formation, and histological staining showed significant increases in the cancellous bone and bone marrow-like tissues. Following the treatment of the pan-Trk inhibitor GNF5837, less ectopic bone was formed and histological analyses also showed fewer amounts of cancellous bone and bone marrow-like tissues compared with those in either the rhNT-3 group or the HO control and saline groups. (Supplementary Fig. 2). Moreover, no significant difference was observed between the HO control and saline treatment either in micro-CT scanning or in histological staining, indicating that the administration of saline around the injured sites may not stimulate HO. Therefore, these data suggested that rhNT-3 treatment significantly alter the ectopic bone volume in injured Achilles tendons.
To further determine functions of rhNT-3 at the injured Achilles tendons, effects on the expression of bone/cartilage-related genes including collagen II, collagen X, Sox9, OCN, Runx2, and OSX were compared among different time points of different treatment groups. The HO group and saline treatment were used as controls. According to the data, there was an active endochondral ossification process at the injured sites. Similar trends were observed for the expression of bone and cartilage-related genes in the saline treatment and the HO groups: the mRNA levels increased since 4 weeks post injury, and peaked at 8 weeks, following a gradually decline until 12 weeks (Fig. 2). In addition, no significant difference was observed between the saline treatment and the HO groups (P > 0.05), implying that the weekly injection of saline did not affect the gene expression in osteogenesis and chondrogenesis.
Effects of treatments with the pan-Trk inhibitor GNF5837 or rhNT-3 on mRNA expression of osteogenesis or chondrogenesis-related genes at injured Achilles tendons. Time-course qPCR analyses for the chondrogenesis-related genes collagen II (a), collagen X (b), and Sox9 (c) as well as the osteogenesis-related genes OCN (d), Runx2 (e) and OSX (F) throughout HO formation. All data represent the mean ± SD. *P < 0.05, **P < 0.005 and ***P < 0.001 versus the HO control group. #P < 0.05, ##P < 0.005, and ###P < 0.001 versus the rhNT-3 group.
For chondrogenesis, rhNT-3 treatment elevated the levels of collagen II (p < 0.001, each time point versus controls), collagen X (p < 0.001, each time point versus controls) and Sox9 (p < 0.001, each time point versus controls), particularly at 8 weeks post operation. Conversely, the pan-Trk inhibitor GNF5837 treatment attenuated the increase in these chondrogenic markers (p < 0.001, each time point of each gene versus controls) at the injured Achilles tendons (Fig. 2a, b, c). For osteogenesis, in comparison with the controls, rhNT-3 treatment enhanced the levels of OCN since 8 weeks (p < 0.001) until 12 weeks (p < 0.001) but had no effects on expression at 4 weeks (p = 0.071) post operation. In addition, rhNT-3 elevated the mRNA levels of Runx2 and OSX from 4 weeks (p < 0.001) through 8 weeks (p < 0.001) post operation, and the expression returned to a lower level at 12 weeks (p < 0.001) (Fig. 2d, e, f). In contrast, the pan-Trk inhibitor GNF5837 treatment significantly alleviated the increase in these bone formation-related genes, including OCN (p < 0.001 for 8 and 12 weeks; p = 0.057), Runx2 (p < 0.001 for all time points) and OSX (p < 0.001 for all time points), compared with rhNT-3 treatment. However, no significant reduction was observed in the levels of OSX in the pan-Trk inhibitor GNF5837 treatment compared with the controls. These data suggested that recombinant NT-3 treatment may markedly stimulating the expression of both bone and cartilage-related genes throughout the formation of HO at the injured Achilles tendons.
Expression of macrophages throughout HO formation at injured Achilles tendons
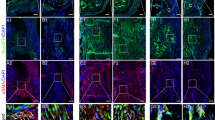
To assess the phenotype and distribution of macrophages at injured Achilles tendons throughout the process of ectopic bone formation, we conducted IHC using a CD68 antibody for the macrophage lineage, iNOS for M1 macrophages, and CD206 for M2 macrophages. The data revealed that CD68+ macrophages were mainly present around the bone marrow-like tissues and persisted through the ossification process (Fig. 3a, a–c). Despite the lack of newly formed cancellous bone and bone marrow-like tissues, cells that stained highly positive for CD68 were more predominant at 4 weeks after operation than at other time points (Fig. 3b), implying that the recruitment of inflammatory macrophages occurred at the early stage of HO formation. However, iNOS+ macrophages were found to a much lesser extent at the injured Achilles tendons compared with CD68+ and CD206+ macrophages (Fig. 3a, d–f). Specifically, the number of M1 macrophages increased at 4 weeks, peaked at 8 weeks, and decreased until 12 weeks (Fig. 3b). In contrast, CD206-positive M2 macrophages were observed in the blood vessels, chondrocytes, osteoblasts lining in the bone marrow-like tissues, and even adipocytes at injured Achilles tendons (Fig. 3a, g–i). Compared with that of CD68+ macrophages, the number of CD206+ macrophages was maintained at a relatively lower level at the early stage of HO and then markedly increased throughout HO formation (Fig. 3b), indicating that M2 macrophages predominated at the late stage of heterotopic bone formation.
a Immunohistochemistry staining of macrophage lineage, M1 and M2 macrophages (brown) at injured Achilles tendons throughout the formation of HO. Scale bar, 100 µm; b Time-course histological analyses of the numbers of positively stained cells at injured Achilles tendons. c Immunofluorescence double-labeling analyses showed the coexpression of NT-3 and macrophages, including M1 and M2 macrophages, at injured Achilles tendons at 12 weeks. Scale bar, 20 µm. B indicates bone, BM indicates bone marrow, C indicates cartilage, black arrows point to vessel endothelial cells, red triangles point to infiltrating mesenchymal cells, black triangles point to chondrocytes, blank triangles points to osteoblasts, and red arrows point to newly formed bone marrow cells. Representative images from one of three experiments are shown (n = 6/group). All data represent the mean ± SD. *P < 0.05, **P < 0.005, and ***P < 0.001 versus the normal control.
To further illustrate the potential relationship between NT-3 and macrophages in the progression of HO formation at injured Achilles tendons, we performed double-labeling IF staining on paraffin sections collected from rats at 12 weeks postoperatively. The data showed the colocalization of NT-3 with the macrophage markers CD68, iNOS, and CD206 around the injured Achilles tendons (Fig. 3c), implying that NT-3 may be derived from macrophages. Thus, these data suggested that macrophages participated in HO formation and may mediate the secretion of NT-3 at the injured Achilles tendons.
RAW264.7 macrophages mediate the secretion of NT-3 in inflammatory conditions
To elucidate the potential source of NT-3 in HO formation in injured Achilles tendons, we simulated the inflammatory environment in vitro using various inflammatory factors and detected the activation of RAW264.7 macrophages and the expression of NT-3. The data showed that LPS, IL-6, or IL-13 induced the phosphorylation of STAT1, STAT3, and STAT6 in a time-dependent manner in RAW264.7 macrophages. The protein expression of P-STAT6 in the LPS group increased after 3 h of treatment; however, P-STAT6 was strongly detected after 6 h of treatment in both the IL-6 and IL-13 groups (Fig. 4a), indicating that LPS had a substantial effect on the induction of RAW264.7 macrophages. In addition, the protein and mRNA levels of iNOS and CD206, which represent M1 and M2 macrophage markers, respectively, were also upregulated in a time-dependent manner when stimulated by LPS, IL-6, and IL-13 (Fig. 4b, c), indicating that either M1 or M2 macrophages were activated in inflammatory conditions. Furthermore, we detected the relatively strong expression of NT-3 in 24 h LPS-treated RAW264.7 macrophages (Fig. 4d) when compared with IL-6- and IL-13-treated cells for 24 h, suggesting that NT-3 may be secreted by macrophages in the inflammatory environment. Therefore, these data suggested that activated macrophages could mediate the secretion of NT-3 in the inflammatory environment in vitro.
a Western blot analyses showed the time-course effects of LPS, IL-6, and IL-13 on the expression of P-STAT1, P-STAT3, and P-STAT6 in RAW264.7 macrophages. b, c Western blot and qPCR analyses showed the time-course effects of LPS, IL-6, and IL-13 on the protein and gene expression of iNOS (M1 macrophage marker) and CD206 (M2 macrophage marker). d Western blot analyses showed NT-3 expression in LPS-, IL-6-, and IL-13-treated RAW264.7 macrophages. All data represent the mean ± SD. *P < 0.05, **P < 0.005, and ***P < 0.001 versus the LPS group.
Effects of NT-3 on osteogenic differentiation of TDSCs in vitro
To explore the potential effects of NT-3 on osteogenesis in vitro, we isolated TDSCs from Achilles tendons and identified them by IF staining with antibodies against SSEA-4 and collagen I (Supplementary Fig. 3B). Before induction of osteogenic differentiation, the cell toxicity of rhNT-3 on proliferation on TDSCs was examined by CCK-8 assays. TDSCs were cultured in the presence of different concentrations of rhNT-3 (1, 10, 100 ng/ml) for 48 h. There was no significant change in cell viability, indicating that the dosage of rhNT-3 (up to 100 ng/ml) had no toxicity on the proliferation of TDSCs (Supplementary Fig. 3C).
Consistent with its positive role in osteogenesis in vivo, rhNT-3 accelerated the mineralization of TDSCs compared with that of the normal osteogenesis group. Increased Alizarin Red staining and a higher Alizarin Red staining absorbance (p < 0.001) were observed in rhNT-3-treated cells. Conversely, co-treatment with the pan-Trk inhibitor GNF5837 reduced the NT-3-induced Alizarin Red staining (rhNT-3+GNF5837 versus rhNT-3 group) and lead to a markedly reduction of the Alizarin Red staining absorbance (p < 0.001) (Fig. 5a and Supplementary Fig. 3D). In addition, rhNT-3 resulted in an enhancement of mRNA expression of Runx2, OCN, and OSX, while the pan-Trk inhibitor GNF5837 attenuated the NT-3-induced levels of these genes in osteogenic cultures of TDSCs (p < 0.005–0.001) (Fig. 5b). Similar protein expression patterns were also observed for Runx2, OCN, and OSX at the time of osteogenesis induction by western blot analyses (Fig. 5c). Consistent with the immunostaining of TrkC at the injured Achilles tendons (Supplementary Fig. 1B), the gene expression of TrkC was elevated throughout the osteogenic differentiation in TDSCs. Furthermore, rhNT-3-treated TDSCs showed a greater induction than the control cells, whereas the pan-Trk inhibitor GNF5837 treatment suppressed the NT-3-induced TrkC expression in osteogenic TDSCs (Fig. 5b, c). Moreover, few significant difference was observed between the control groups and GNF5837 groups either in cell staining or in expressions of mRNA and protein. Taken together, these data suggested that NT-3 had osteogenic effects on TDSCs in vitro and the pan-Trk inhibitor GNF5837 could suppress NT-3-induced osteogenesis in TDSCs.
a Representative images of Alizarin Red staining of rhNT-3-treated cells in osteogenic culture after 21 days compared with control group, rhNT-3 + pan-Trk inhibitor GNF5837 group and GNF5837 group. The absorbance of Alizarin Red staining at 490 nm is shown. b Time-course expression of mRNA levels of TrkC, Runx2, OCN, and OSX during osteogenic differentiation in the rhNT-3, GNF5837, rhNT-3+GNF5837, and control groups. c Time-course expression of Runx2, OCN, and OSX protein levels during osteogenic differentiation in rhNT-3, GNF5837, rhNT-3 + GNF5837 and the control groups. d Representative western blot detecting the expression of phosphorylated (p) and total (t) ERK1/2 in TDSCs with rhNT-3 or PD98059 using GAPDH as a loading control. e Representative western blot detecting the expression of phosphorylated (p) and total (t) PI3K and Akt in TDSCs with rhNT-3 or LY294002 using GAPDH as a loading control. All data represent the mean ± SD. *P < 0.05, **P < 0.005, and ***P < 0.001 versus the HO control group. #P < 0.05, ##P < 0.005 and, ###P < 0.001 versus the rhNT-3 group.
To further elucidate the potential mechanisms of NT-3 in the osteogenic differentiation of TDSCs in vitro, we selected PD98059, an inhibitor of the EKR1/2 signaling pathway, and LY294002, an inhibitor of PI3K/Akt signaling pathway. Protein expression of t-ERK1/2, PI3K, and t-Akt as well as phosphorylated-ERK1/2, PI3K, and Akt were enhanced in rhNT-3-treated TDSCs compared with untreated cells (Fig. 5d, e). However, rhNT-3-induced enhancement of t-ERK1/2 and its phosphorylated form were partially inhibited by PD98059 (Fig. 5d). Similarly, rhNT-3-activated PI3K/Akt pathway was partially inhibited by LY294002 (Fig. 5e). These data suggested that NT-3 may promote osteogenesis of TDSCs through activating phosphorylation of the ERK1/2 and PI3K/Akt signaling pathways.
Macrophages depletion decrease NT-3 expression and HO formation at injured Achilles tendons
To determine the expression of NT-3 during HO formation, we measured the concentration of NT-3 in the serum from HO rats at the indicated time points using normal rats as a control. Significantly elevated levels of serum NT-3 were observed in the different stages of HO formation and were distinctly higher than those of the normal control (Fig. 6a). Furthermore, we evaluated the secretion of NT-3 in the culture medium of RAW264.7 macrophages under the inflammatory environment by ELISA. The data showed that NT-3 was upregulated in a time manner under the inflammatory environment at different time points in each group, showing the highest level in LPS-treated groups (Fig. 6b), which was consistent with previous data in Fig. 5d. These results confirmed that NT-3 could be secreted by macrophages under inflammation in vitro.
a Concentration of serum NT-3 from normal rats or rats with injured Achilles tendons at different time points. b Concentration of NT-3 in the culture medium of RAW264.7 macrophages under the treatment of LPS, IL-6, and IL-13. c HO volumes at injured Achilles tendons after micro-CT 3D reconstruction for PBS-liposomes group and clodronate–liposomes group at 12 weeks. d Concentration of serum NT-3 from PBS-liposomes group and clodronate–liposomes group with injured Achilles tendons at different time points. e Time-course gene expression of NT-3 from PBS-liposomes group and clodronate–liposomes group at injured Achilles tendons (fold changes versus PBS-liposomes group). All data represent the mean ± SD. *P < 0.05, **P < 0.005 and ***P < 0.001 versus PBS-liposomes group.
To further investigate the importance of macrophages to expression of NT-3 and HO formation at injured Achilles tendons, rats underwent Achilles tenotomy were injected intravenously with clodronate–liposomes to deplete macrophages, using PBS-liposomes as controls. Micro-CT showed that clodronate-loaded liposomes significantly reduced the HO volume when compared with the control group (Fig. 6c). The concentration of serum NT-3 in clodronate–liposomes groups were markedly lower than that of in PBS-liposomes groups during HO pathogenesis (Fig. 6d). Similarly, the data showed a decreased the mRNA levels of NT-3 in clodronate–liposomes groups in comparison with the controls at injured Achilles tendons during HO formation (Fig. 6e). Collectively, these results suggest that macrophages may have a profound role in expression of NT-3 and ectopic bone formation at injured Achilles tendons.
Discussion
The pathophysiology of HO, with the proposed theory of endochondral ossification [25], remains largely unknown. Despite previous studies describing the effects of NTs on bone (particularly in fracture healing) [26,27,28], the potential roles of NTs in HO formation remain unclear. NT-3, one of the members of the NT family, is regarded as a critical trophic factor involved in the regulation of development, survival, and function of the nervous system [29]. However, the function of NT-3 has been proven to extend beyond its original role in fracture healing, bony repair of injured growth plates, and wound repair [11, 14, 30].
In the previous study, we observed that NT-3 was found to enhance osteogenesis and promote ectopic bone formation at injured Achilles tendons [15]. In the current study, we demonstrated that NT-3 is important for surrounding mesenchymal cells such as TDSCs to participate in HO formation at injury sites. Our data showed that NT-3 and TrkC were highly induced than other NTs and receptors, suggesting that NT-3 may have a role in HO formation. In addition, the administration of exogenous NT-3 led to increased levels of expression in both osteogenesis-related and chondrogenesis-related genes at injured Achilles tendons throughout the formation of HO. According to our data, the expression of Sox9, Collagen II and X, markers for chondrogenesis, were upregulated during chondrogenetic phase from 4 to 8 weeks and downregulated during osteogenic phase at 12 weeks. The osteogenic marker OCN was increased from 4 to 12 weeks during HO pathological process while Runx2 and OSX were upregulated from 4 to 8 weeks and returned to a lower level at 12 weeks. The “bell curve” patterns of expression of bone/cartilage-related genes were in consistent with corresponding stages (chondrogenesis, osteogenesis, and maturation) of endochondral ossification process as previously described in Achilles tenotomy model [16].
A major challenge in studying the NT-3-TrkC signaling pathway is the lack of receptor-specific inhibitors. In this study, we have used the small pan-Trk inhibitor molecule GNF5837 to investigate the function of NT-3-TrkC signaling pathway in HO [22]. NT-3 treatment could promote ectopic bone formation, while the pan-Trk inhibitor GNF5837 treatment, immunoneutralization of endogenous NT-3, obviously attenuated HO formation at injured Achilles tendons and reduced the elevation of bone and cartilage-related genes. Consistent with our in vivo studies, NT-3 showed osteogenic effects in TDSCs, while the pan-Trk inhibitor GNF5837 exhibited the rescue effects on NT-3-induced osteogenesis of TDSCs, indicating that NT-3 promoted osteogenic differentiation of TDSCs and inhibition of NT-3 by GNF5837 could suppress osteogenesis of TDSCs in vitro. These results suggest that NT-3-TrkC signaling pathway may participate in regulating HO formation.
Although the current signaling pathways of NTs are mainly documented in studies of the nervous system, increasing evidence now demonstrates that NTs-Trk signaling pathways also exert their effects in the skeletal system [7, 8, 31]. A recent study demonstrated that NT-3-induced activation of TrkC in BMSCs resulted in rapid phosphorylation of ERK1/2 and PI3K/Akt, which are major kinases downstream of the Trk receptor signaling pathway in neuronal cells [32]. Similarly, ERK1/2 activation was also determined in BDNF-stimulated cementoblasts and NT-4-treated periodontal ligament cells [33,34,35]. In addition, previous studies have demonstrated that ERK1/2 and PI3K/Akt signaling pathways were rapidly activated by treatment with NT-3 in an injured growth plate model [10, 11]. Consistent with these previous studies, our in vitro studies showed that NT-3 treatment caused activation of TrkC and phosphorylation of ERK1/2 and PI3K/Akt in TDSCs. Furthermore, PD98059, a specific inhibitor of the ERK1/2 signaling pathway [9], prohibited NT-3-induced phosphorylation of ERK1/2, and suppressed osteogenic differentiation of TDSCs. In addition, LY294002, an inhibitor of PI3K/Akt signaling pathway [36], showed the similar suppression on phosphorylation of PI3K/Akt and osteogenesis of TDSCs. These findings suggested that the osteogenic function of NT-3 in this study may be at least partly modulated by the ERK1/2 and PI3K/Akt signaling pathways.
The first step of HO formation is local inflammation. The presence of inflammatory cells, including macrophages, lymphocytes, and mast cells, in the perivascular region of early HO lesions is associated with recruitment of the undifferentiated progenitor cells [17, 37, 38]. In addition to host–pathogen interactions, inflammation and wounds healing, these immune cells also have a primary role in bone remodeling and repair, which is largely associated with the inflammatory response following bone injuries. Macrophages serve as a regulator of the inflammatory response and can be divided into M1 and M2 subphenotypes based on cell surface markers [39, 40]. Several studies reported the crucial role of macrophages in HO formation in mice, particularly in the initiation stage, as selective depletion of macrophages in genetically altered mice significantly prohibited ectopic bone formation [18, 41]. Furthermore, recent studies showed that macrophages were present and persist throughout the endochondral ossification process [37, 42, 43], suggesting the critical role that macrophages play in HO formation. In this study, we observed that macrophages, including M1 and M2, were expressed at the injured Achilles tendon sites throughout the HO formation (Fig. 3a), supporting the idea that macrophages participated in the process of HO formation. In addition, we analyzed the time-course expression of M1 and M2 macrophages, and found that M1 macrophages highly induced at the early stage (4 weeks) of HO formation, and gradually returned a lower level in late stage (12 weeks). In contrast, the antiinflammatory M2 showed a rising expression from 4 to 12 weeks throughout the HO pathology process (Fig. 3b). Innovatively, our double-labeling immunostaining studies revealed the colocalization of NT-3 and macrophages at injured Achilles tendons during HO formation, indicating that NT-3 may be derived from macrophages. These findings were supported by previous work showing the expression of NGF and BDNF by macrophages in human and animal studies [21].
The inflammatory response induces a cascade of cytokines, which in turn promote angiogenesis and induce osteoprogenitor cells to release BMPs, promoting osteogenic differentiation [17]. Previous studies reported that the inflammatory markers IL-3, IL-6, effluent IL-10, and effluent IL-13 were associated with HO, indicating that the inflammatory response in general was important in the formation of HO [44, 45]. Evans et al. [46] have found that a severe systemic and wound-specific inflammatory state as evident by elevated levels of inflammatory cytokines is associated with the development of HO, suggesting serum IL-6, IL-10, and MCP-1, as well as wound effluent IL-10 and MIP-1 cytokine were associated with the development of HO. Similarly, Forsberg et al. [45] have demonstrated that inflammatory markers IL-3, IL 12p70, effluent IL-3, and effluent IL-13 were associated with HO. Furthermore, previous study [44] has identified TLR4, which has been linked to bone mass, specifically LPS-induced bone resorption, as being associated with the formation of HO. Accordingly, our in vitro studies demonstrated that stimulation with inflammatory factors, such as LPS, IL-6, and IL-13, caused phosphorylation of STAT1/3/6, indicating the activation of macrophages. Then, we detected NT-3 expression in LPS-activated macrophages, whereas NT-3 expression was rare in both the IL-6 and IL-13 treatment groups. In addition, NT-3 expression was upregulated in a time- and dose-dependent manner in LPS-treated macrophages, suggesting that activated macrophages may be the source of NT-3. These findings support the idea that NT-3 plays a role in promoting HO through the macrophage-mediated paracrine pathway. Furthermore, depletion of macrophages led to the reduction of HO formation, as well as decrease of NT-3 secretion and expression at injured Achilles tendons, supporting the hypothesis that the inflammatory macrophages may be the resources of the injury site-derived NT-3 in the development of ectopic bone formation.
Although vascular invasion is a critical step for initiation of HO formation [38], one limitation of this study was the relatively few studies of NT-3-stimulated angiogenesis in HO formation. However, the observation of the immunolocalization of NT-3 in newly formed blood vessels demonstrated that NT-3 may play an essential role in angiogenesis, and future studies are required to examine the direct effects of NT-3 on the vascular system. In addition, another limitation of this study is that, owing to the current lack of TrkC-specific inhibitors, the effect of NT-3 on HO formation may not be clearly and robustly verified since we employed the pan-Trk inhibitor. Although the negative effect of the pan-Trk inhibitor GNF5837 on HO formation in vivo is consistent with our previous finding that treatment with the nonspecific TrkC inhibitor could reduce ectopic bone formation in vivo by suppressing NT-3-induced endothelial–mesenchymal transition (EndMT) in rat endothelial cells [15], we still may not directly draw the conclusions that NT-3 could be the primary driver in HO formation in vivo and future studies are still needed. Our main strength was that we utilized relatively simple and repeatable animal models to demonstrate the effects and potential mechanisms of NT-3 on HO. More importantly, we described the underlying relationship between injury site-derived NT-3 and macrophages in HO formation.
In summary, the current study demonstrates that macrophage-derived NT-3 may promote pathogenesis of HO formation in injured Achilles tendons and these effects are modulated partly by the ERK1/2 and PI3K/Akt-activated signaling pathways. This study also identified the potential origin of NT-3 and confirmed the essential roles of macrophages in HO formation in injured Achilles tendons, which may shed light on therapeutic directions and strategies for HO in the future.
References
Legosz P, Drela K, Pulik L, Sarzynska S, Maldyk P. Challenges of heterotopic ossification-molecular background and current treatment strategies. Clin Exp Pharmacol Physiol. 2018;45:1229–35.
Nauth A, Giles E, Potter BK, Nesti LJ, O'Brien FP, Bosse MJ, et al. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26:684–8.
Wang X, Li F, Xie L, Crane J, Zhen G, Mishina Y, et al. Inhibition of overactive TGF-beta attenuates progression of heterotopic ossification in mice. Nat Commun. 2018;9:551.
Ranganathan K, Loder S, Agarwal S, Wong VW, Forsberg J, Davis TA, et al. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am. 2015;97:1101–11.
Davies OG, Grover LM, Eisenstein N, Lewis MP, Liu Y. Identifying the cellular mechanisms leading to heterotopic ossification. Calcif Tissue Int. 2015;97:432–44.
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell. Signal. 2011;23:609–20.
Mitre M, Mariga A, Chao MV. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin Sci. 2017;131:13–23.
Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64.
Su YW, Zhou XF, Foster BK, Grills BL, Xu J, Xian CJ. Roles of neurotrophins in skeletal tissue formation and healing. J Cell Physiol. 2018;233:2133–45.
Su YW, Chim SM, Zhou L, Hassanshahi M, Chung R, Fan C, et al. Osteoblast derived-neurotrophin3 induces cartilage removal proteases and osteoclast-mediated function at injured growth plate in rats. Bone. 2018;116:232–47.
Su YW, Chung R, Ruan CS, Chim SM, Kuek V, Dwivedi PP, et al. Neurotrophin-3 induces BMP-2 and VEGF activities and promotes the bony repair of injured growth plate cartilage and bone in rats. J Bone Miner Res. 2016;31:1258–74.
Asaumi K, Nakanishi T, Asahara H, Inoue H, Takigawa M. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone. 2000;26:625–33.
Aiga A, Asaumi K, Lee YJ, Kadota H, Mitani S, Ozaki T, et al. Expression of neurotrophins and their receptors tropomyosin-related kinases (Trk) under tension-stress during distraction osteogenesis. Acta Med Okayama. 2006;60:267–77.
Li X, Sun DC, Li Y, Wu XY. Neurotrophin-3 improves fracture healing in rats. Eur Rev Med Pharmacol Sci. 2018;22:2439–46.
Zhang J, Wang L, Cao H, Chen N, Yan B, Ao X, et al. Neurotrophin-3 acts on the endothelial–mesenchymal transition of heterotopic ossification in rats. J Cell Mol Med. 2019;23:2595–609.
Lin L, Shen Q, Xue T, Yu C. Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: expression of bone and cartilage related genes. Bone. 2010;46:425–31.
Kraft CT, Agarwal S, Ranganathan K, Wong VW, Loder S, Li J, et al. Trauma-induced heterotopic bone formation and the role of the immune system: a review. J Trauma Acute Care Surg. 2016;80:156–65.
Genet F, Kulina I, Vaquette C, Torossian F, Millard S, Pettit AR, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol. 2015;236:229–40.
Torossian F, Guerton B, Anginot A, Alexander KA, Desterke C, Soave S, et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI insight. 2017;2:e9604323.
Zhao J, Wang L, Li Y. Electroacupuncture alleviates the inflammatory response via effects on M1 and M2 macrophages after spinal cord injury. Acupunct Med. 2017;35:224–30.
Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583–9.
Albaugh P, Fan Y, Mi Y, Sun F, Adrian F, Li N, et al. Discovery of GNF-5837, a selective TRK inhibitor with efficacy in rodent cancer tumor models. ACS Med Chem Lett. 2012;3:140–5.
Jiang H, Chen Y, Chen G, Tian X, Tang J, Luo L, et al. Leptin accelerates the pathogenesis of heterotopic ossification in rat tendon tissues via mTORC1 signaling. J Cell Physiol. 2018;223:1017–28.
Lui PP, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev Rep. 2011;7:883–97.
Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem cells. 2009;27:150–6.
Kilian O, Hartmann S, Dongowski N, Karnati S, Baumgart-Vogt E, Hartel FV, et al. BDNF and its TrkB receptor in human fracture healing. Ann Anat. 2014;196:286–95.
Tomlinson RE, Li Z, Zhang Q, Goh BC, Li Z, Thorek DLJ, et al. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 2016;16:2723–35.
Akiyama Y, Mikami Y, Watanabe E, Watanabe N, Toriumi T, Takahashi T, et al. The P75 neurotrophin receptor regulates proliferation of the human MG63 osteoblast cell line. Differentiation. 2014;87:111–8.
Yan YH, Li SH, Gao Z, Zou SF, Li HY, Tao ZY, et al. Neurotrophin-3 promotes proliferation and cholinergic neuronal differentiation of bone marrow-derived neural stem cells via notch signaling pathway. Life Sci. 2016;166:131–8.
Shen L, Zeng W, Wu YX, Hou CL, Chen W, Yang MC, et al. Neurotrophin-3 accelerates wound healing in diabetic mice by promoting a paracrine response in mesenchymal stem cells. Cell Transplant. 2013;22:1011–21.
Rezaee F, Rellick SL, Piedimonte G, Akers SM, O'Leary HA, Martin K, et al. Neurotrophins regulate bone marrow stromal cell IL-6 expression through the MAPK pathway. PloS ONE. 2010;5:e9690.
Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Target. 2008;7:46–62.
Kajiya M, Shiba H, Fujita T, Takeda K, Uchida Y, Kawaguchi H, et al. Brain-derived neurotrophic factor protects cementoblasts from serum starvation-induced cell death. J Cell Physiol. 2009;221:696–706.
Kajiya M, Shiba H, Fujita T, Ouhara K, Takeda K, Mizuno N, et al. Brain-derived neurotrophic factor stimulates bone/cementum-related protein gene expression in cementoblasts. J Biol Chem. 2008;283:16259–67.
Mizuno N, Shiba H, Inui T, Takeda K, Kajiya M, Hasegawa N, et al. Effect of neurotrophin-4/5 on bone/cementum-related protein expressions and DNA synthesis in cultures of human periodontal ligament cells. J Periodontol. 2008;79:2182–9.
Lin C, Shao Y, Zeng C, Zhao C, Fang H, Wang L, et al. Blocking PI3K/AKT signaling inhibits bone sclerosis in subchondral bone and attenuates post-traumatic osteoarthritis. J Cell Physiol. 2018;233:6135–47.
Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78–89.
Hackett L, Millar NL, Lam P, Murrell GA. Are the symptoms of calcific tendinitis due to neoinnervation and/or neovascularization? J Bone Joint Surg Am. 2016;98:186–92.
Horwood NJ. Macrophage polarization and bone formation: a review. Clin Rev Allergy Immunol. 2016;51:79–86.
Sinder BP, Pettit AR, McCauley LK. Macrophages: their emerging roles in bone. J Bone Miner Res. 2015;30:2140–9.
Convente MR, Chakkalakal SA, Yang E, Caron RJ, Zhang D, Kambayashi T, et al. Depletion of mast cells and macrophages impairs heterotopic ossification in an Acvr1(R206H) mouse model of fibrodysplasia ossificans progressiva. J Bone Miner Res. 2018;33:269–82.
Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184:3192–204.
Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30:1090–102.
Mitchell EJ, Canter J, Norris P, Jenkins J, Morris J. The genetics of heterotopic ossification: insight into the bone remodeling pathway. J Orthop Trauma. 2010;24:530–3.
Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014;472:2845–54.
Evans KN, Forsberg JA, Potter BK, Hawksworth JS, Brown TS, Andersen R, et al. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma. 2012;26:e2 04–e213.
Acknowledgements
We thank Xin Yue and Guangpu Yang (Department of Medical Science Experimental Center, Sun Yat-Sen University) for the technical support. A special thank-you is also extended to Nan Chen for her valuable help during my study and life. This study was supported by grants from the National Natural Science Foundation of China (No. 81672228, No. 81702199, and No. 31801012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, L., Chu, J. et al. Macrophage-derived neurotrophin-3 promotes heterotopic ossification in rats. Lab Invest 100, 762–776 (2020). https://doi.org/10.1038/s41374-019-0367-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-019-0367-x
This article is cited by
-
The Role of Neuromodulation and Potential Mechanism in Regulating Heterotopic Ossification
Neurochemical Research (2024)
-
Inhibition of NF-κB Signaling-Mediated Crosstalk Between Macrophages and Preosteoblasts by Metformin Alleviates Trauma-Induced Heterotopic Ossification
Inflammation (2023)
-
Meniscus repair via collagen matrix wrapping and bone marrow injection: clinical and biomolecular study
International Orthopaedics (2023)
-
Macrophage-Derived Extracellular DNA Initiates Heterotopic Ossification
Inflammation (2023)
-
Macrophages in heterotopic ossification: from mechanisms to therapy
npj Regenerative Medicine (2021)