当前位置:
X-MOL 学术
›
Microchim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amperometric hydrazine sensor based on the use of a gold nanoparticle-modified nanocomposite consisting of porous polydopamine, multiwalled carbon nanotubes and reduced graphene oxide
Microchimica Acta ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1007/s00604-019-4014-4 Xinjin Zhang 1 , Jianbin Zheng 1
Microchimica Acta ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1007/s00604-019-4014-4 Xinjin Zhang 1 , Jianbin Zheng 1
Affiliation
|
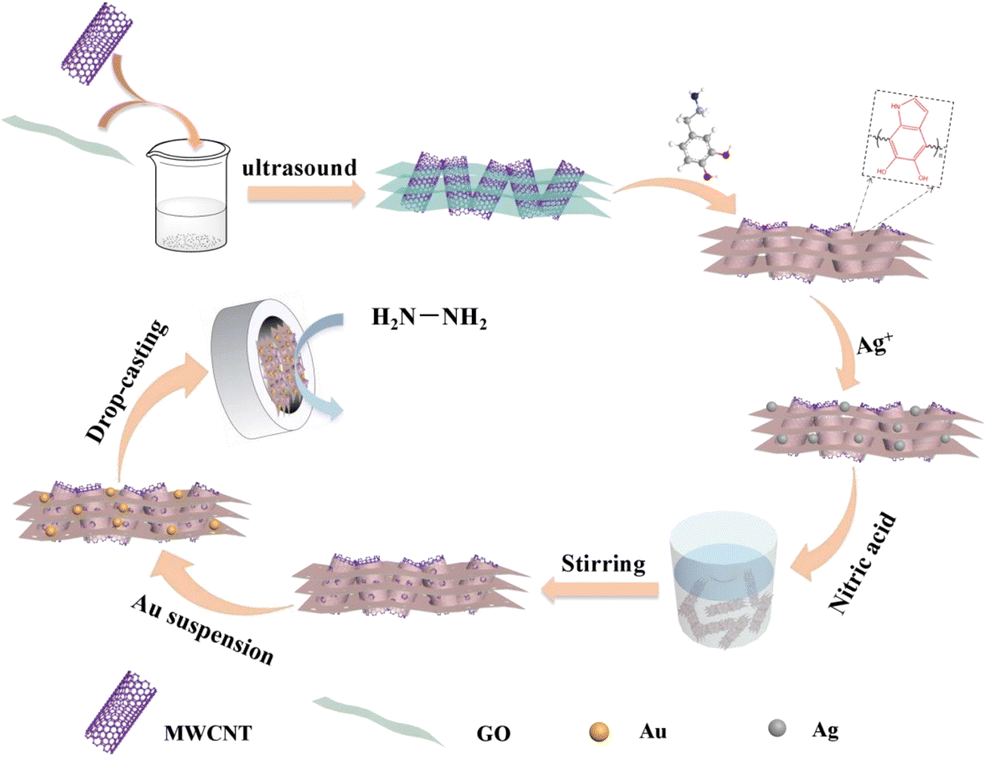
A porous hybrid material was prepared from polydopamine-modified multiwalled carbon nanotubes and reduced graphene oxide. It was employed as a supporting material for an electrochemical hydrazine sensor. Gold nanoparticles with a size of about 13 nm were placed on the material which then was characterized by transmission electron microscopy, field emission-scanning electron microscopy, Raman spectra, FTIR and nitrogen absorption/desorption plots. The material is highly porous and has a specific surface of 290 m 2 g −1 , which is larger than that of P-MWCNT/rGO alone (149 m 2 g −1 ), and an increased pore volume. It was placed on a glassy carbon electrode (GCE), and cyclic voltammetry, chronoamperometry and amperometric i-t curves were used to characterize the catalytic activity of the sensor. The kinetic parameters of the modified GCE were calculated which proved that it has a high catalytic efficiency in promoting the electron transfer kinetics of hydrazine. The amperometric signal (obtained at a typical working potential of 0.35 V vs. SCE) has two linear ranges, one from 1 μM - 3 mM and one from 3 to 55 mM, with sensitivities of 524 and 98 A mM −1 cm −2 , respectively. The detection limit is 0.31 μM. Graphical abstract The porous nanocomposite was synthesized by etching silver nanoparticles and a enhanced non-enzymatic electrochemical sensor of hydrazine was successfully designed. The electrochemical performances of the modified electrode were also examined.
中文翻译:

基于使用由多孔聚多巴胺、多壁碳纳米管和还原氧化石墨烯组成的金纳米颗粒改性纳米复合材料的电流型肼传感器
由聚多巴胺改性的多壁碳纳米管和还原氧化石墨烯制备多孔杂化材料。它被用作电化学肼传感器的支撑材料。将尺寸约为 13 nm 的金纳米粒子置于材料上,然后通过透射电子显微镜、场发射扫描电子显微镜、拉曼光谱、FTIR 和氮吸收/解吸图对其进行表征。该材料具有高度多孔性,比表面积为 290 m 2 g -1 ,比单独使用 P-MWCNT/rGO 的比表面积(149 m 2 g -1 )大,并且孔体积增加。将其置于玻碳电极 (GCE) 上,并使用循环伏安法、计时电流法和电流法 it 曲线来表征传感器的催化活性。计算了改性GCE的动力学参数,证明其在促进肼电子转移动力学方面具有较高的催化效率。电流测量信号(在 0.35 V 与 SCE 的典型工作电位下获得)具有两个线性范围,一个从 1 μM - 3 mM 和一个从 3 到 55 mM,灵敏度分别为 524 和 98 A mM -1 cm -2 , 分别。检测限为 0.31 μM。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。电流测量信号(在 0.35 V 与 SCE 的典型工作电位下获得)具有两个线性范围,一个从 1 μM - 3 mM 和一个从 3 到 55 mM,灵敏度分别为 524 和 98 A mM -1 cm -2 , 分别。检测限为 0.31 μM。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。电流测量信号(在 0.35 V 与 SCE 的典型工作电位下获得)具有两个线性范围,一个从 1 μM - 3 mM 和一个从 3 到 55 mM,灵敏度分别为 524 和 98 A mM -1 cm -2 , 分别。检测限为 0.31 μM。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。
更新日期:2020-01-01
中文翻译:

基于使用由多孔聚多巴胺、多壁碳纳米管和还原氧化石墨烯组成的金纳米颗粒改性纳米复合材料的电流型肼传感器
由聚多巴胺改性的多壁碳纳米管和还原氧化石墨烯制备多孔杂化材料。它被用作电化学肼传感器的支撑材料。将尺寸约为 13 nm 的金纳米粒子置于材料上,然后通过透射电子显微镜、场发射扫描电子显微镜、拉曼光谱、FTIR 和氮吸收/解吸图对其进行表征。该材料具有高度多孔性,比表面积为 290 m 2 g -1 ,比单独使用 P-MWCNT/rGO 的比表面积(149 m 2 g -1 )大,并且孔体积增加。将其置于玻碳电极 (GCE) 上,并使用循环伏安法、计时电流法和电流法 it 曲线来表征传感器的催化活性。计算了改性GCE的动力学参数,证明其在促进肼电子转移动力学方面具有较高的催化效率。电流测量信号(在 0.35 V 与 SCE 的典型工作电位下获得)具有两个线性范围,一个从 1 μM - 3 mM 和一个从 3 到 55 mM,灵敏度分别为 524 和 98 A mM -1 cm -2 , 分别。检测限为 0.31 μM。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。电流测量信号(在 0.35 V 与 SCE 的典型工作电位下获得)具有两个线性范围,一个从 1 μM - 3 mM 和一个从 3 到 55 mM,灵敏度分别为 524 和 98 A mM -1 cm -2 , 分别。检测限为 0.31 μM。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。电流测量信号(在 0.35 V 与 SCE 的典型工作电位下获得)具有两个线性范围,一个从 1 μM - 3 mM 和一个从 3 到 55 mM,灵敏度分别为 524 和 98 A mM -1 cm -2 , 分别。检测限为 0.31 μM。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。图形摘要 通过蚀刻银纳米粒子合成了多孔纳米复合材料,并成功设计了一种增强型肼非酶电化学传感器。还检查了修饰电极的电化学性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号