Abstract
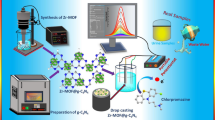
A porous hybrid material was prepared from polydopamine-modified multiwalled carbon nanotubes and reduced graphene oxide. It was employed as a supporting material for an electrochemical hydrazine sensor. Gold nanoparticles with a size of about 13 nm were placed on the material which then was characterized by transmission electron microscopy, field emission-scanning electron microscopy, Raman spectra, FTIR and nitrogen absorption/desorption plots. The material is highly porous and has a specific surface of 290 m2 g−1, which is larger than that of P-MWCNT/rGO alone (149 m2 g−1), and an increased pore volume. It was placed on a glassy carbon electrode (GCE), and cyclic voltammetry, chronoamperometry and amperometric i-t curves were used to characterize the catalytic activity of the sensor. The kinetic parameters of the modified GCE were calculated which proved that it has a high catalytic efficiency in promoting the electron transfer kinetics of hydrazine. The amperometric signal (obtained at a typical working potential of 0.35 V vs. SCE) has two linear ranges, one from 1 μM - 3 mM and one from 3 to 55 mM, with sensitivities of 524 and 98 A mM−1 cm−2, respectively. The detection limit is 0.31 μM.

The porous nanocomposite was synthesized by etching silver nanoparticles and a enhanced non-enzymatic electrochemical sensor of hydrazine was successfully designed. The electrochemical performances of the modified electrode were also examined.








Similar content being viewed by others
References
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56
Wu J, Hu F, Hu X, Wei Z, Shen PK (2008) Improved kinetics of methanol oxidation on Pt/hollow carbon sphere catalysts. Electrochim Acta 53:8341–8345
Wang C, Zhang L, Guo Z, Xu J, Wang H, Zhai K, Zhuo X (2010) A novel hydrazine electrochemical sensor based on the high specific surface area graphene. Microchim Acta 169:1–6
Cumings J, Zettl A (2000) Low-friction nanoscale linear bearing realized from multiwall carbon nanotubes. Science 289:602–604
Zhang LL, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38:2520–2531
Zhang Y, Ren J, Zhao Y, Tan T, Yin F, Wang Y (2019) A porous 3D-RGO@ MWCNT hybrid material as Li-S battery cathode. Beilstein J Nanotech 10:514–521
Fang B, Feng Y, Liu M, Wang G, Zhang X, Wang M (2011) Electrocatalytic oxidation of hydrazine at a glassy carbon electrode modified with nickel ferrite and multi-walled carbon nanotubes. Microchim Acta 175:145–150
Chaban VV, Prezhdo OV (2011) Water boiling inside carbon nanotubes: toward efficient drug release. ACS Nano 5:5647–5655
Hashwan SSB, Ruslinda AR, Fatin MF, Arshad MM, Hashim U (2017) Reduced graphene oxide-multiwalled carbon nanotubes composites as sensing membrane electrodes for DNA detection. Microsyst Technol 23:3421–3428
Cote LJ, Kim J, Tung VC, Luo J, Kim F, Huang J (2010) Graphene oxide as surfactant sheets. Pure Appl Chem 83:95–110
Veerapandian M, Lee MH, Krishnamoorthy K, Yun K (2012) Synthesis, characterization and electrochemical properties of functionalized graphene oxide. Carbon 50:4228–4238
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Qiu L, Yang X, Gou X, Yang W, Ma ZF, Wallace GG, Li D (2010) Dispersing carbon nanotubes with graphene oxide in water and synergistic effects between graphene derivatives. Chem-Eur J 16:10653–10658
Zhang C, Ren L, Wang X, Liu T (2010) Graphene oxide-assisted dispersion of pristine multiwalled carbon nanotubes in aqueous media. J Phys Chem C 114:11435–11440
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430
Ye W, Hu H, Zhang H, Zhou F, Liu W (2010) Multi-walled carbon nanotube supported Pd and Pt nanoparticles with high solution affinity for effective electrocatalysis. Appl Surf Sci 256:6723–6728
Liu Y, Ai K, Liu J, Deng M, He Y, Lu L (2013) Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater 25:1353–1359
Ryu J, Ku SH, Lee H, Park CB (2010) Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv Funct Mater 20:2132–2139
Yang Z, Qi C, Zheng X, Zheng J (2016) Sensing hydrogen peroxide with a glassy carbon electrode modified with silver nanoparticles, AlOOH and reduced graphene oxide. Microchim Acta 183:1131–1136
Zhao Y, Wang C, Liu J, Wang F (2018) PDA-assisted formation of ordered intermetallic CoPt3 catalysts with enhanced oxygen reduction activity and stability. Nanoscale 10:9038–9043
Gu M, Sui Q, Farooq U, Zhang X, Qiu Z, Lyu S (2018) Degradation of phenanthrene in sulfate radical based oxidative environment by nZVI-PDA functionalized rGO catalyst. Chem Eng J 354:541–552
Yang Z, Sheng Q, Zhang S, Zheng X, Zheng J (2017) One-pot synthesis of Fe3O4/polypyrrole/graphene oxide nanocomposites for electrochemical sensing of hydrazine. Microchim Acta 184:2219–2226
Zhu Y, Chandra P, Shim YB (2012) Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine-au nanoparticle-aptamer bioconjugate. Anal Chem 85:1058–1064
Rahman MM, Alfonso VG, Fabregat-Santiago F, Bisquert J, Asiri AM, Alshehri AA, Albar HA (2017) Hydrazine sensors development based on a glassy carbon electrode modified with a nanostructured TiO2 films by electrochemical approach. Microchim Acta 184:2123–2129
Choudhary G, Hansen H (1998) Human health perspective of environmental exposure to hydrazines: a review. Chemosphere 37:801–843
Rahman MM, Khan A, Marwani HM, Asiri AM (2016) Hydrazine sensor based on silver nanoparticle-decorated polyaniline tungstophosphate nanocomposite for use in environmental remediation. Microchim Acta 183:1787–1796
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11:771–778
Frens G (1972) Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nature 241:20–22
Zhan W, Gao L, Fu X, Siyal SH, Sui G, Yang X (2019) Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal. Appl Surf Sci 467:1122–1133
Liu Y, Liu X, Guo Z, Hu Z, Xue Z, Lu X (2017) Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens Bioelectron 87:101–107
Bard AJ, Faulkner LR (2001) Fundamentals and applications. Electrochemical Methods 2:482
Li J, Xie H, Chen L (2011) A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sensor Actuat B: Chem 153:239–245
Wang G, Zhang C, He X, Li Z, Zhang X, Wang L, Fang B (2010) Detection of hydrazine based on Nano-Au deposited on Porous-TiO2 film. Electrochim Acta 55:7204–7210
Samadi-Maybodi A, Ghasemi S, Ghaffari-Rad H (2015) A novel sensor based on Ag-loaded zeolitic imidazolate framework-8 nanocrystals for efficient electrocatalytic oxidation and trace level detection of hydrazine. Sensor Actuat B: Chem 220:627–633
Lee KK, Loh PY, Sow CH, Chin WS (2013) CoOOH nanosheet electrodes: simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine. Biosens Bioelectron 39:255–260
Shi E, Lin H, Wang Q, Zhang F, Shi S, Zhang T, Qu F (2017) Synergistic effect of the composite films formed by zeolitic imidazolate framework 8 (ZIF-8) and porous nickel films for enhanced amperometric sensing of hydrazine. Dalton T 46:554–563
Aziz MA, Kawde AN (2013) Gold nanoparticle-modified graphite pencil electrode for the high-sensitivity detection of hydrazine. Talanta 115:214–221
Chen W, Wang H, Tang H, Yang C, Guan X, Li Y (2019) Amperometric sensing of hydrazine by using single gold nanopore electrodes filled with Prussian blue and coated with polypyrrole and carbon dots. Microchim Acta 186:350
Salimi A, Miranzadeh L, Hallaj R (2008) Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 75:147–156
Hamidi H, Bozorgzadeh S, Haghighi B (2017) Amperometric hydrazine sensor using a glassy carbon electrode modified with gold nanoparticle-decorated multiwalled carbon nanotubes. Microchim Acta 184:4537–4543
Acknowledgments
The authors gratefully acknowledge the financial support of this project by the National Science Foundation of China (No.21575113), Northwest University Graduate Innovation and Creativity Funds (No.YZZ17125), and the Natural Science Foundation of Shaanxi Province in China (No. 2017JM2036, 2018JQ2029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 237 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Zheng, J. Amperometric hydrazine sensor based on the use of a gold nanoparticle-modified nanocomposite consisting of porous polydopamine, multiwalled carbon nanotubes and reduced graphene oxide. Microchim Acta 187, 89 (2020). https://doi.org/10.1007/s00604-019-4014-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4014-4