当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual redox stability of pentavalent uranium with hetero-bifunctional phosphonocarboxylate: insight into aqueous speciation
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-03-26 , DOI: 10.1039/d4dt00173g Ashutosh Srivastava 1 , Sk Musharaf Ali 2 , Rama Mohan Rao Dumpala 1 , Sumit Kumar 3 , Pranaw Kumar 4 , Neetika Rawat 1 , P K Mohapatra 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-03-26 , DOI: 10.1039/d4dt00173g Ashutosh Srivastava 1 , Sk Musharaf Ali 2 , Rama Mohan Rao Dumpala 1 , Sumit Kumar 3 , Pranaw Kumar 4 , Neetika Rawat 1 , P K Mohapatra 1
Affiliation
|
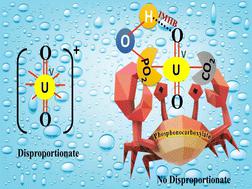
The +5 state is an unusual oxidation state of uranium due to its instability in the aqueous phase. As a result, gaining information about its aqueous speciation is extremely difficult. The present work is an attempt in that direction and it provides insight into the existence of a new pentavalent species in the presence of hetero-bifunctional phosphonocarboxylate (PC) chelators, other than the carbonate ion, in the aqueous medium. The aqueous chemistry of pentavalent uranium species with three environmentally relevant PCs was probed using electrochemical and DFT methods to understand the redox energy and kinetics of conversion of the U(VI)/U(V) couple, stability, structure, stoichiometry, binding modes, etc. Interestingly, pentavalent uranium complexes with PCs are quite persistent over a wide range of pH starting from acidic to alkaline conditions. The PC chelators block the cation–cation interaction (CCI) of U(V) through strong hetero-bidentate chelation and intermolecular hydrogen bonding (IMHB) interactions which stabilize the pentavalent metal ion against disproportionation. For uranyl species in the presence of PCs, acting as chelators, CV plots were obtained at varying pH values from 2 to 8. The obtained results indicate an irreversible single redox peak involving U(VI) to U(V) conversion and association of a coupled chemical reaction with the electron transfer step. ESI-MS studies were performed to understand the speciation effect on the U(VI)/U(V) redox couple with varying pH. Speciation modelling of U(V) with the PC ligands was carried out, which indicated that the U(V) is redox stable in nearly 47% of the pH region in the presence of the PCs as compared to the carboxylate-based chelators. The free energy and reduction potential of the U(V) complexes and the reduction free energy and disproportionation free energy for the U(VI)/U(V) couple were determined by DFT computations in the presence of the PCs. In situ spectroelectrochemical spectra were recorded to provide evidence for the existence of U(V) species with PCs in the aqueous medium and to acquire its absorption spectra. The present study is highly significant for understanding the coordination chemistry of pentavalent uranium species, accurate modelling of uranium, and isolation of U(V).
中文翻译:

五价铀与异双功能膦酰基羧酸盐的异常氧化还原稳定性:洞察水相形态
+5 态是铀的一种不寻常的氧化态,因为它在水相中不稳定。因此,获得有关其水相形态的信息极其困难。目前的工作是朝这个方向的尝试,它提供了对在水介质中除碳酸根离子之外的异双功能膦酰基羧酸盐 (PC) 螯合剂存在下新五价物质的存在的见解。使用电化学和 DFT 方法探讨了五价铀物种与三种环境相关 PC 的水化学,以了解 U( VI )/U( V ) 对的氧化还原能和转化动力学、稳定性、结构、化学计量、结合模式、 ETC 。有趣的是,五价铀与 PC 的配合物在从酸性到碱性条件的广泛 pH 范围内都相当持久。 PC 螯合剂通过强异二齿螯合和分子间氢键 (IMHB) 相互作用来阻断 U( V ) 的阳离子-阳离子相互作用 (CCI),从而稳定五价金属离子,防止歧化。对于 PC 存在下的铀酰物质,作为螯合剂,在 2 至 8 的不同 pH 值下获得了 CV 图。获得的结果表明,涉及 U( VI ) 到 U( V ) 转化和关联的不可逆单一氧化还原峰将化学反应与电子转移步骤耦合。进行 ESI-MS 研究是为了了解不同 pH 值对 U( VI )/U( V ) 氧化还原对的形态影响。 对 U( V ) 与 PC 配体进行了形态建模,结果表明,与基于羧酸盐的螯合剂相比,在 PC 存在的情况下,U( V ) 在近 47% 的 pH 范围内是氧化还原稳定的。 U( V )配合物的自由能和还原势以及U( VI )/U( V )对的还原自由能和歧化自由能在PC存在的情况下通过DFT计算确定。记录原位光谱电化学光谱,为水介质中 U( V ) 物种与 PC 的存在提供证据,并获取其吸收光谱。本研究对于理解五价铀物种的配位化学、铀的精确建模以及U( V )的分离具有非常重要的意义。
更新日期:2024-03-26
中文翻译:

五价铀与异双功能膦酰基羧酸盐的异常氧化还原稳定性:洞察水相形态
+5 态是铀的一种不寻常的氧化态,因为它在水相中不稳定。因此,获得有关其水相形态的信息极其困难。目前的工作是朝这个方向的尝试,它提供了对在水介质中除碳酸根离子之外的异双功能膦酰基羧酸盐 (PC) 螯合剂存在下新五价物质的存在的见解。使用电化学和 DFT 方法探讨了五价铀物种与三种环境相关 PC 的水化学,以了解 U( VI )/U( V ) 对的氧化还原能和转化动力学、稳定性、结构、化学计量、结合模式、 ETC 。有趣的是,五价铀与 PC 的配合物在从酸性到碱性条件的广泛 pH 范围内都相当持久。 PC 螯合剂通过强异二齿螯合和分子间氢键 (IMHB) 相互作用来阻断 U( V ) 的阳离子-阳离子相互作用 (CCI),从而稳定五价金属离子,防止歧化。对于 PC 存在下的铀酰物质,作为螯合剂,在 2 至 8 的不同 pH 值下获得了 CV 图。获得的结果表明,涉及 U( VI ) 到 U( V ) 转化和关联的不可逆单一氧化还原峰将化学反应与电子转移步骤耦合。进行 ESI-MS 研究是为了了解不同 pH 值对 U( VI )/U( V ) 氧化还原对的形态影响。 对 U( V ) 与 PC 配体进行了形态建模,结果表明,与基于羧酸盐的螯合剂相比,在 PC 存在的情况下,U( V ) 在近 47% 的 pH 范围内是氧化还原稳定的。 U( V )配合物的自由能和还原势以及U( VI )/U( V )对的还原自由能和歧化自由能在PC存在的情况下通过DFT计算确定。记录原位光谱电化学光谱,为水介质中 U( V ) 物种与 PC 的存在提供证据,并获取其吸收光谱。本研究对于理解五价铀物种的配位化学、铀的精确建模以及U( V )的分离具有非常重要的意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号