RSC主编推荐:有机领域精彩文章快览(免费阅读原文)
英国皇家化学会(RSC)是一个拥有175年历史的面向全球化学家的非营利会员制机构,旗下拥有43种期刊,其中很多在化学领域有很高影响力。为了进一步帮助广大读者追踪科技前沿热点,X-MOL团队与英国皇家化学会合作,推出英国皇家化学会期刊主编推荐的精彩文章快览,本期文章属“有机领域”,英文点评来自英国皇家化学会期刊的主编。如果大家对我们的解读有更多的补充和点评,欢迎在文末写评论发表您的高见!
Organic Chemistry Frontiers (IF: 4.955)

1. Photoredox meets gold Lewis acid catalysis in the alkylative semipinacol rearrangement: a photocatalyst with a dark side
Org. Chem. Front., 2017, Advance Article
DOI: 10.1039/C7QO00590C
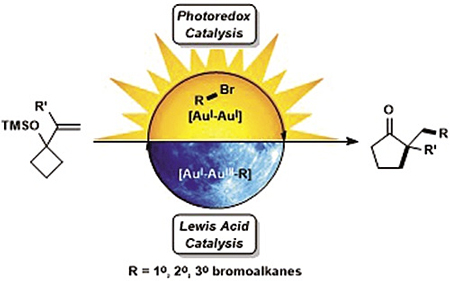
The alkylative semipinacol rearrangement of a variety of TMS protected α-styrenyl substituted cyclic alcohols with unactivated bromoalkanes that merge photoredox and Au(I)/Au(III) catalysis has been achieved. This redox neutral rearrangement is marked by a dimeric Au(I) photocatalyst that plays two roles; photoredox activation of bromoalkanes and Au(III)-mediated semipinacol rearrangement coupled with C(sp3)–C(sp3) reductive elimination; a reaction mode rarely accessed. This operationally simple methodology contains readily available starting materials that undergo reaction with [Au2(dppm)2]Cl2 upon irradiation with UVA LEDs, furnishing diversified ketone products. Primary, secondary, and tertiary bromoalkanes and a range of TMS protected α-styrenyl substituted alcohols were investigated in this transformation.
加拿大渥太华大学的研究者通过非活化的溴代烷烃在光氧化还原和Au(I)/Au(III)催化作用下反应,实现了多种α-苯乙烯基环醇类化合物的烷基半频哪醇重排反应。这种氧化还原中性的重排反应由发挥两种作用的二聚体Au(I)光催化剂引发。其反应模式十分少见,包括光氧化还原活化溴代烷烃、Au(III)介导的半频哪醇重排以及C(sp3)–C(sp3)还原消除反应。这种操作简单的方法只需要在[Au2(dppm)2]Cl2的存在下,将易得的原料置于UVA LED灯下照射便可生成多种酮类产物。文中还讨论了多种一级、二级、三级溴代烷烃和一系列TMS保护的α-苯乙烯基取代的醇在该类转化中的作用。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Palladium catalyzed C(sp3)–H acetoxylation of aliphatic primary amines to γ-amino alcohol derivatives
Org. Chem. Front., 2017, Advance Article
DOI: 10.1039/C7QO00432J

It still remains a major challenge to apply free primary amino groups as the directing group for aliphatic C–H functionalization. In this article, the authors used the protonation strategy to control the binding ability of primary amines and realized free amino group directed inert aliphatic C–H acetoxylation in good chemo- and regio-selectivity. This methodology provided a straightforward approach from primary amines to γ-amino alcohols.
将游离的伯氨基团作为脂肪族C-H键官能团化反应中的导向基团仍然是一项重大的挑战。北京大学的施章杰教授课题组通过质子化策略来调控伯氨的结合能力,实现了高化学与区域选择性的游离氨基导向的惰性脂肪族C-H键乙酰氧基化反应。该工作提供了一种伯氨直接转化为γ-氨基醇的方法。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

Chemical Science (IF: 8.668)

1. Photocatalytic oxidation of benzene to phenol using dioxygen as an oxygen source and water as an electron source in the presence of a cobalt catalyst
Chem. Sci., 2017, Advance Article
DOI: 10.1039/C7SC02495A

Scientists in Japan and Korea have reported for the first time an example of photocatalytic hydroxylation of benzene with dioxygen and water, to afford a high turnover number of over 500. This study showed that benzene is oxidized to phenol by O2 under visible light irradiation of a reaction solution containing water as an electron source in the presence of a cobalt catalyst. This work has provided a new way towards direct oxygenation of substrates by O2 and water.
来自韩国和日本的研究者首次通过O2和水实现了苯的光催化羟基化反应,其转换数超过500。研究结果表明,在可见光及钴催化剂的作用下,苯可在水作为电子来源的反应溶液中被O2氧化为苯酚。该工作为O2和水直接参与底物的氧化提供了新的研究策略。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Nickel-catalyzed asymmetric hydrogenation of β-acylamino nitroolefins: an efficient approach to chiral amines
Chem. Sci., 2017, 8, 6419-6422
DOI: 10.1039/C7SC02669B

Chinese researchers have developed an efficient approach for synthesizing chiral β-amino nitroalkanes. This was achieved using the Ni-catalyzed asymmetric hydrogenation of β-acylamino nitroolefins using H2 as the reductant. This catalytic system was carried out under mild conditions, and the desired products were obtained in excellent yields and with high enantioselectivities.
中国多家研究机构的研究者合作报道了一种有效合成手性β-氨基硝基烷烃的方法。他们以氢气作为还原剂,实现了镍催化β-酰胺基硝基烯烃的不对称氢化反应。该催化反应可以在温和的条件下进行,以优秀的产率与高对映选择性得到目标产物。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!












































 京公网安备 11010802027423号
京公网安备 11010802027423号