Chemical Physics Letters ( IF 2.8 ) Pub Date : 2019-01-30 , DOI: 10.1016/j.cplett.2019.01.015 Emilie Bak Pedersen , Simone Thirstrup Andersen , Ole John Nielsen
|
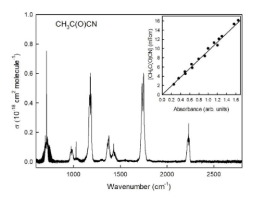
The atmospheric chemistry of pyruvonitrile, CH3C(O)CN, was studied experimentally using FTIR smog chamber techniques. All experiments were performed at 296 ± 2 K and 700 Torr. The rate constants k(CH3C(O)CN + Cl) = (1.01 ± 0.26) × 10-14 cm3 molecule-1 s-1 and k(CH3C(O)CN + OH) = (3.02 ± 1.11) × 10-13 cm3 molecule-1 s-1 were determined using the relative rate method. NCC(O)OONO2, ClC(O)OONO2, CO and HCN were identified as products from the atmospheric degradation of CH3C(O)CN. The atmospheric lifetime of CH3C(O)CN was calculated to 38 days and GWP100 to 1.
中文翻译:

CH 3 C(O)CN的大气化学:与Cl原子和OH自由基的动力学和反应机理
使用FTIR烟雾室技术对丙酮腈CH 3 C(O)CN的大气化学进行了实验研究。所有实验均在296±2 K和700托下进行。速率常数k(CH 3 C(O)CN + Cl)=(1.01±0.26)×10 -14 cm 3分子-1 s -1,k(CH 3 C(O)CN + OH)=(3.02± 1.11)×10 -13 cm 3分子-1 s -1用相对速率法测定。NCC(O)OONO 2,ClC(O)OONO 2,CO和HCN被确定为CH 3大气降解的产物C(O)CN。CH 3 C(O)CN的大气寿命经计算为38天,GWP为100。











































 京公网安备 11010802027423号
京公网安备 11010802027423号