当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genesis and Stability of Hydronium Ions in Zeolite Channels
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-30 , DOI: 10.1021/jacs.8b07969 Meng Wang 1 , Nicholas R Jaegers 1, 2 , Mal-Soon Lee 1 , Chuan Wan 1 , Jian Zhi Hu 1 , Hui Shi 1 , Donghai Mei 1 , Sarah D Burton 1 , Donald M Camaioni 1 , Oliver Y Gutiérrez 1 , Vassiliki-Alexandra Glezakou 1 , Roger Rousseau 1 , Yong Wang 1, 2 , Johannes A Lercher 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-30 , DOI: 10.1021/jacs.8b07969 Meng Wang 1 , Nicholas R Jaegers 1, 2 , Mal-Soon Lee 1 , Chuan Wan 1 , Jian Zhi Hu 1 , Hui Shi 1 , Donghai Mei 1 , Sarah D Burton 1 , Donald M Camaioni 1 , Oliver Y Gutiérrez 1 , Vassiliki-Alexandra Glezakou 1 , Roger Rousseau 1 , Yong Wang 1, 2 , Johannes A Lercher 1, 3
Affiliation
|
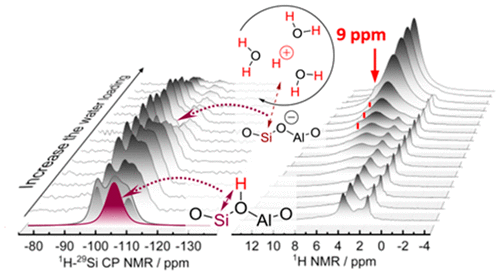
The catalytic sites of acidic zeolite are profoundly altered by the presence of water changing the nature of the Brønsted acid site. High-resolution solid-state NMR spectroscopy shows water interacting with zeolite Brønsted acid sites, converting them to hydrated hydronium ions over a wide range of temperature and thermodynamic activity of water. A signal at 9 ppm was observed at loadings of 2-9 water molecules per Brønsted acid site and is assigned to hydrated hydronium ions on the basis of the evolution of the signal with increasing water content, chemical shift calculations, and the direct comparison with HClO4 in water. The intensity of 1H-29Si cross-polarization signal first increased and then decreased with increasing water chemical potential. This indicates that hydrogen bonds between water molecules and the tetrahedrally coordinated aluminum in the zeolite lattice weaken with the formation of hydronium ion-water clusters and increase the mobility of protons. DFT-based ab initio molecular dynamics studies at multiple temperatures and water concentrations agree well with this interpretation. Above 140 °C, however, fast proton exchange between bridging hydroxyl groups and water occurs even in the presence of only one water molecule per acid site.
中文翻译:

水合氢离子在沸石通道中的成因和稳定性
酸性沸石的催化位点因水的存在而发生深刻变化,从而改变了布朗斯台德酸性位点的性质。高分辨率固态 NMR 光谱显示水与沸石布朗斯台德酸位相互作用,在很宽的温度和水的热力学活性范围内将它们转化为水合水合氢离子。在每个 Brønsted 酸位点加载 2-9 个水分子时观察到 9 ppm 的信号,并根据信号随含水量增加的演变、化学位移计算以及与 HClO4 的直接比较,将其指定为水合水合氢离子在水里。1H-29Si交叉极化信号的强度随着水化学势的增加先增大后减小。这表明水分子与沸石晶格中四面体配位铝之间的氢键随着水合氢离子-水簇的形成而减弱,并增加了质子的迁移率。在多个温度和水浓度下基于 DFT 的 ab initio 分子动力学研究与这种解释非常吻合。然而,在 140 °C 以上,即使在每个酸位点只有一个水分子的情况下,桥接羟基和水之间也会发生快速质子交换。
更新日期:2019-01-30
中文翻译:

水合氢离子在沸石通道中的成因和稳定性
酸性沸石的催化位点因水的存在而发生深刻变化,从而改变了布朗斯台德酸性位点的性质。高分辨率固态 NMR 光谱显示水与沸石布朗斯台德酸位相互作用,在很宽的温度和水的热力学活性范围内将它们转化为水合水合氢离子。在每个 Brønsted 酸位点加载 2-9 个水分子时观察到 9 ppm 的信号,并根据信号随含水量增加的演变、化学位移计算以及与 HClO4 的直接比较,将其指定为水合水合氢离子在水里。1H-29Si交叉极化信号的强度随着水化学势的增加先增大后减小。这表明水分子与沸石晶格中四面体配位铝之间的氢键随着水合氢离子-水簇的形成而减弱,并增加了质子的迁移率。在多个温度和水浓度下基于 DFT 的 ab initio 分子动力学研究与这种解释非常吻合。然而,在 140 °C 以上,即使在每个酸位点只有一个水分子的情况下,桥接羟基和水之间也会发生快速质子交换。











































 京公网安备 11010802027423号
京公网安备 11010802027423号