当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparison between Batch and Continuous Monoclonal Antibody Production and Economic Analysis
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2019-02-13 , DOI: 10.1021/acs.iecr.8b04717 Ou Yang 1 , Siddharth Prabhu 1 , Marianthi Ierapetritou 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2019-02-13 , DOI: 10.1021/acs.iecr.8b04717 Ou Yang 1 , Siddharth Prabhu 1 , Marianthi Ierapetritou 1
Affiliation
|
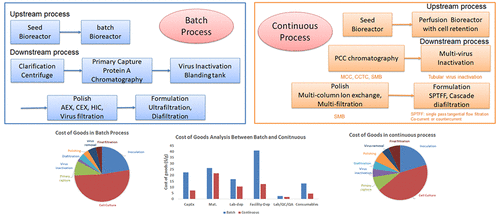
This work aims to evaluate the benefits of the continuous process in biopharmaceutical manufacturing of monoclonal antibodies (mAbs). An integrated continuous process is designed and built using a process simulator and compared with a fed-batch process production line. The comparisons are based on cost of goods (COG/g) calculation and sensitivity analysis. The fed-batch process results in operating COG/g of $99/g in mAbs production, whereas the continuous process accounts for $51/g. Because of the smaller footprint and fewer storage tanks required in the continuous process, the facility cost reduced by 66%, compared to the fed-batch process. In continuous production, there is a better utilization of the primary capture resin, which leads to a reduction in consumables cost by 68%. Sensitivity analysis is used to evaluate the process in different manufacturing scales (50–1200 kg/yr), upstream titers (1.5–5.5 g/L), and downstream yield (70%–80%). Cost savings can be observed through the entire range.
中文翻译:

分批和连续单克隆抗体生产的比较及经济性分析
这项工作旨在评估连续过程在生物制药生产单克隆抗体(mAb)中的益处。使用过程模拟器设计和构建集成的连续过程,并与分批补料生产线进行比较。比较是基于商品成本(COG / g)计算和敏感性分析。补料分批工艺产生的单克隆抗体生产中的运营COG / g为$ 99 / g,而连续工艺的成本为$ 51 / g。由于连续过程所需的占地面积较小且所需的储罐较少,因此与分批进料过程相比,设备成本降低了66%。在连续生产中,可以更好地利用初级捕集树脂,从而将耗材成本降低68%。敏感性分析用于评估不同制造规模(50–1200 kg /年),上游效价(1.5–5.5 g / L)和下游产量(70%–80%)的过程。可以在整个范围内节省成本。
更新日期:2019-02-14
中文翻译:

分批和连续单克隆抗体生产的比较及经济性分析
这项工作旨在评估连续过程在生物制药生产单克隆抗体(mAb)中的益处。使用过程模拟器设计和构建集成的连续过程,并与分批补料生产线进行比较。比较是基于商品成本(COG / g)计算和敏感性分析。补料分批工艺产生的单克隆抗体生产中的运营COG / g为$ 99 / g,而连续工艺的成本为$ 51 / g。由于连续过程所需的占地面积较小且所需的储罐较少,因此与分批进料过程相比,设备成本降低了66%。在连续生产中,可以更好地利用初级捕集树脂,从而将耗材成本降低68%。敏感性分析用于评估不同制造规模(50–1200 kg /年),上游效价(1.5–5.5 g / L)和下游产量(70%–80%)的过程。可以在整个范围内节省成本。



























 京公网安备 11010802027423号
京公网安备 11010802027423号