Biochimie ( IF 3.3 ) Pub Date : 2019-01-31 , DOI: 10.1016/j.biochi.2019.01.019 Madhuparna Bose , Sudipta Bhattacharyya , Rupam Biswas , Amlan Roychowdhury , Atanu Bhattacharjee , Ananta Kumar Ghosh , Amit Kumar Das
|
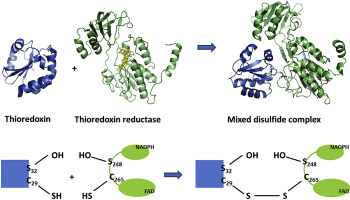
The redox homeostasis of cytoplasm is maintained by a series of disulfide exchange reactions mediated by proteins belonging to the thioredoxin superfamily. Thioredoxin and thioredoxin reductase, being the major members of the family, play a key role in oxidative stress response of Staphylococcus aureus. In this report, we have identified and characterised an active thioredoxin system of the mentioned pathogen. Crystal structure of thioredoxin2 (SaTrx2) in its reduced form reveals that it contains the conserved redox active WCXXC motif and a thioredoxin fold. Thioredoxin reductase2 (SaTR2) is a flavoprotein and consists of two Rossmann folds as the binding sites for FAD and NADPH. Crystal structure of the SaTR2 holoenzyme shows that the protein consists of two domains and the catalytic site comprises of an intramolecular disulfide bond formed between two sequentially distal cysteine residues. Biophysical and biochemical studies unveil that SaTrx2 and SaTR2 can physically interact in solution and in the course of sustaining the redox equilibrium, the latter reduces the former. Molecular docking has been performed to illustrate the interface formed between SaTrx2 and SaTR2 during the disulfide exchange reaction.
中文翻译:

阐明葡萄球菌硫氧还蛋白2和硫氧还蛋白还原酶2之间的二硫键交换的机制:结构的见解。
细胞质的氧化还原稳态通过一系列属于硫氧还蛋白超家族的蛋白质介导的二硫键交换反应来维持。硫氧还蛋白和硫氧还蛋白还原酶是该家族的主要成员,在金黄色葡萄球菌的氧化应激反应中起关键作用。在本报告中,我们已经鉴定并鉴定了所述病原体的活性硫氧还蛋白系统。还原形式的硫氧还蛋白2(SaTrx2)的晶体结构表明,它包含保守的氧化还原活性WCXXC基序和硫氧还蛋白折叠。硫氧还蛋白还原酶2(SaTR2)是一种黄素蛋白,由两个罗斯曼折叠组成,是FAD和NADPH的结合位点。SaTR2全酶的晶体结构表明,该蛋白质由两个结构域组成,催化位点由在两个顺序的远端半胱氨酸残基之间形成的分子内二硫键组成。生物物理和生化研究表明,SaTrx2和SaTR2可以在溶液中发生物理相互作用,并且在维持氧化还原平衡的过程中,后者会降低前者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号