Oncogene ( IF 6.9 ) Pub Date : 2019-01-30 , DOI: 10.1038/s41388-019-0680-2 Ben Yi Tew , Christophe Legendre , Gerald C. Gooden , Kyle N. Johnson , Rae Anne Martinez , Jeff Kiefer , Mark Bernstein , Jennifer Glen , Loren Butry , Aleksander Hinek , Steven A. Toms , Bodour Salhia
|
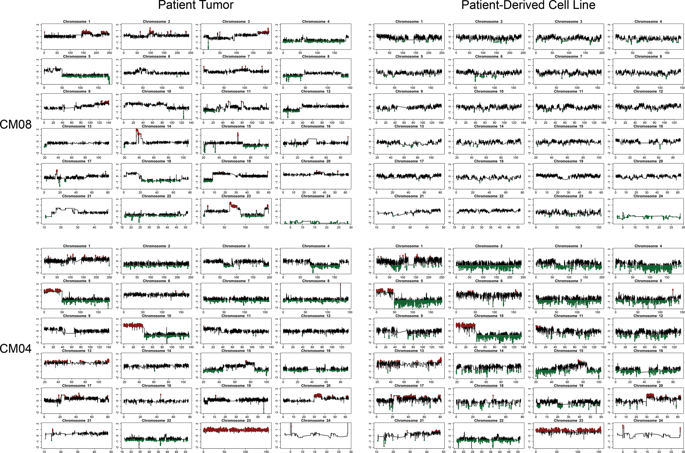
The functional role of human derived stromal cells in the tumor microenviornment of CNS metastases (CM) remain understudied. The purpose of the current study was to isolate and characterize stromal cells of the tumor microenvironment in CM. Four different patient-derived cell lines (PDCs) of stromal and one PDC of tumorigenic origin were generated from breast or lung CM. PDCs were analyzed by DNA/RNA sequencing, DNA methylation profiling, and immunophenotypic assays. The stromal derived PDCs were termed CNS metastasis-associated stromal cells (cMASCs). Functional analysis of cMASCs was tested by co-implanting them with tumorigenic cells in mice. cMASCs displayed normal genotypes compared with tumorigenic cell lines. RNA-seq and DNA methylation analyses demonstrated that cMASCs highly resembled each other, suggesting a common cell of origin. Additionally, cMASCs revealed gene expression signatures associated with cancer associated fibroblasts (CAFs), epithelial to mesenchymal transition, mesenchymal stem cells and expressed high levels of collagen. Functionally, cMASCs restricted tumor growth, and induced desmoplasia in vivo, suggesting that cMASCs may promote a protective host response to impede tumor growth. In summary, we demonstrated the isolation, molecular characterization and functional role of human derived cMASCs, a subpopulation of cells in the microenvironment of CM that have tumor inhibitory functions.
中文翻译:

患者来源的CNS转移相关基质细胞系的分离和鉴定
人源性基质细胞在中枢神经系统转移瘤(CM)的肿瘤微环境中的功能作用仍未得到研究。本研究的目的是分离和表征CM中肿瘤微环境的基质细胞。从乳腺或肺部CM产生了基质的四种不同的患者来源细胞系(PDC)和致瘤性的一种PDC。通过DNA / RNA测序,DNA甲基化分析和免疫表型分析来分析PDC。基质衍生的PDC被称为CNS转移相关基质细胞(cMASC)。通过将cMASCs与致瘤细胞共同植入小鼠体内,测试了cMASCs的功能分析。与致瘤细胞系相比,cMASCs显示出正常的基因型。RNA-seq和DNA甲基化分析表明,cMASC彼此高度相似,表明是共同的起源细胞。此外,cMASCs还揭示了与癌症相关的成纤维细胞(CAF),上皮向间充质转化,间充质干细胞相关的基因表达特征,并表达了高水平的胶原蛋白。在功能上,cMASCs限制了肿瘤的生长,并在体内诱导了增生,提示cMASCs可能促进保护性宿主反应,从而阻止肿瘤的生长。总之,我们证明了人源性cMASCs的分离,分子表征和功能作用,这是CM微环境中具有肿瘤抑制功能的细胞亚群。并在体内诱发了异型增生,提示cMASCs可能促进保护性宿主反应,从而阻止肿瘤的生长。总之,我们证明了人源性cMASCs的分离,分子表征和功能作用,这是CM微环境中具有肿瘤抑制功能的细胞亚群。并在体内诱发了异型增生,提示cMASCs可能促进保护性宿主反应,从而阻止肿瘤的生长。总之,我们证明了人源性cMASCs的分离,分子表征和功能作用,这是CM微环境中具有肿瘤抑制功能的细胞亚群。









































 京公网安备 11010802027423号
京公网安备 11010802027423号