Oncogene ( IF 6.9 ) Pub Date : 2019-01-29 , DOI: 10.1038/s41388-019-0704-y Shuo Fang , Ming Liu , Lei Li , Fei-Fei Zhang , Yun Li , Qian Yan , Yu-Zhu Cui , Ying-Hui Zhu , Yun-Fei Yuan , Xin-Yuan Guan
|
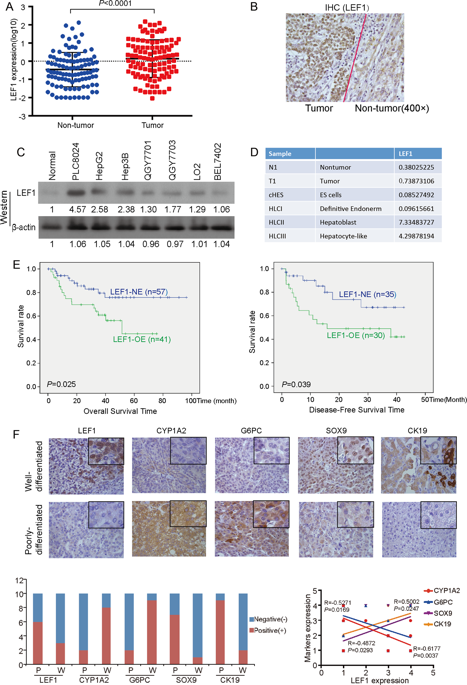
The poorly differentiated hepatocellular carcinoma (HCC) cells are usually characterized by immature hepatic progenitor cell-like properties, such as enhanced self-renewal ability, resistance to chemotherapeutic drugs, and a loss of mature hepatocyte proteins. However, the molecular mechanisms governing this process still remain unclear. In this study, we found the lymphoid enhancer-binding factor-1 (LEF1), a transcriptional factor, was frequently overexpressed in HCCs, which was significantly associated with poor prognosis and tumor cell differentiation. Functional studies have found that LEF1 enhanced cell growth, foci formation, colony formation in soft agar, and tumor formation in nude mice. Different from its canonical roles in the WNT signaling pathway, we found that LEF1 could activate the critical members (e.g., NOTCH1 and NOTCH2) of the NOTCH signaling pathway through directly binding to their promoter regions. Further studies have found that LEF1 could enhance the self-renewal ability, drug resistance, dedifferentiation, and invasion of HCC cells. The oncogenic functions and the effects of LEF1 on cancer stemness could be effectively inhibited by NOTCH inhibitor. Further characterization of LEF1 may lead to the development of novel therapeutic strategies for HCC treatment.
中文翻译:

淋巴增强剂结合因子-1通过直接激活NOTCH途径促进肝细胞癌的干性和较差的分化
低分化肝细胞癌(HCC)细胞通常以未成熟的肝祖细胞样特性为特征,例如增强的自我更新能力,对化疗药物的抗性以及成熟肝细胞蛋白的丢失。但是,控制这一过程的分子机制仍然不清楚。在这项研究中,我们发现淋巴增强因子结合因子1(LEF1),一种转录因子,在肝癌中经常过表达,这与不良预后和肿瘤细胞分化显着相关。功能研究发现,LEF1增强了细胞生长,病灶形成,软琼脂中的集落形成以及裸鼠中的肿瘤形成。与它在WNT信号通路中的典型作用不同,我们发现LEF1可以通过直接结合它们的启动子区域来激活NOTCH信号传导途径的关键成员(例如,NOTCH1和NOTCH2)。进一步的研究发现,LEF1可以增强HCC细胞的自我更新能力,耐药性,去分化和侵袭能力。NOTCH抑制剂可有效抑制其致癌功能和LEF1对癌干的作用。LEF1的进一步表征可能会导致HCC治疗的新型治疗策略的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号