Immunity ( IF 25.5 ) Pub Date : 2019-01-29 , DOI: 10.1016/j.immuni.2018.12.020 María Martínez-López 1 , Salvador Iborra 2 , Ruth Conde-Garrosa 1 , Annalaura Mastrangelo 1 , Camille Danne 3 , Elizabeth R Mann 4 , Delyth M Reid 5 , Valérie Gaboriau-Routhiau 6 , Maria Chaparro 7 , María P Lorenzo 8 , Lara Minnerup 9 , Paula Saz-Leal 1 , Emma Slack 10 , Benjamin Kemp 11 , Javier P Gisbert 7 , Andrzej Dzionek 9 , Matthew J Robinson 11 , Francisco J Rupérez 8 , Nadine Cerf-Bensussan 12 , Gordon D Brown 5 , David Bernardo 7 , Salomé LeibundGut-Landmann 13 , David Sancho 1
|
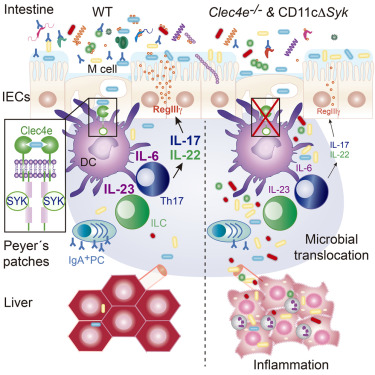
Production of interleukin-17 (IL-17) and IL-22 by T helper 17 (Th17) cells and group 3 innate lymphoid cells (ILC3s) in response to the gut microbiota ensures maintenance of intestinal barrier function. Here, we examined the mechanisms whereby the immune system detects microbiota in the steady state. A Syk-kinase-coupled signaling pathway in dendritic cells (DCs) was critical for commensal-dependent production of IL-17 and IL-22 by CD4+ T cells. The Syk-coupled C-type lectin receptor Mincle detected mucosal-resident commensals in the Peyer’s patches (PPs), triggered IL-6 and IL-23p19 expression, and thereby regulated function of intestinal Th17- and IL-17-secreting ILCs. Mice deficient in Mincle or with selective depletion of Syk in CD11c+ cells had impaired production of intestinal RegIIIγ and IgA and increased systemic translocation of gut microbiota. Consequently, Mincle deficiency led to liver inflammation and deregulated lipid metabolism. Thus, sensing of commensals by Mincle and Syk signaling in CD11c+ cells reinforces intestinal immune barrier and promotes host-microbiota mutualism, preventing systemic inflammation.
中文翻译:

树突状细胞中 Mincle-Syk 轴的微生物群感应调节 IL-17 和 -22 的产生并促进肠道屏障的完整性
辅助 T 17 (Th17) 细胞和第 3 组先天淋巴细胞 (ILC3) 响应肠道微生物群而产生白细胞介素 17 (IL-17) 和 IL-22,确保维持肠道屏障功能。在这里,我们研究了免疫系统检测稳态微生物群的机制。树突状细胞 (DC) 中的 Syk 激酶偶联信号通路对于 CD4 + T 细胞共生依赖性产生 IL-17 和 IL-22 至关重要。 Syk 偶联的 C 型凝集素受体 Mincle 检测派尔氏淋巴结 (PP) 中的粘膜驻留共生体,触发 IL-6 和 IL-23p19 表达,从而调节肠道分泌 Th17 和 IL-17 的 ILC 的功能。 Mincle 缺陷或 CD11c +细胞中 Syk 选择性缺失的小鼠肠道 RegIIIγ 和 IgA 的产生受损,肠道微生物群的系统易位增加。因此,Mincle 缺乏会导致肝脏炎症和脂质代谢失调。因此,CD11c +细胞中 Mincle 和 Syk 信号传导对共生体的感知增强了肠道免疫屏障,促进宿主-微生物群互利共生,防止全身炎症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号