Oncogene ( IF 6.9 ) Pub Date : 2019-01-28 , DOI: 10.1038/s41388-019-0709-6 Bo Tang , Jilin Wu , Michael X. Zhu , Xuemei Sun , Jingjing Liu , Rui Xie , Tobias Xiao Dong , Yufeng Xiao , John M. Carethers , Shiming Yang , Hui Dong
|
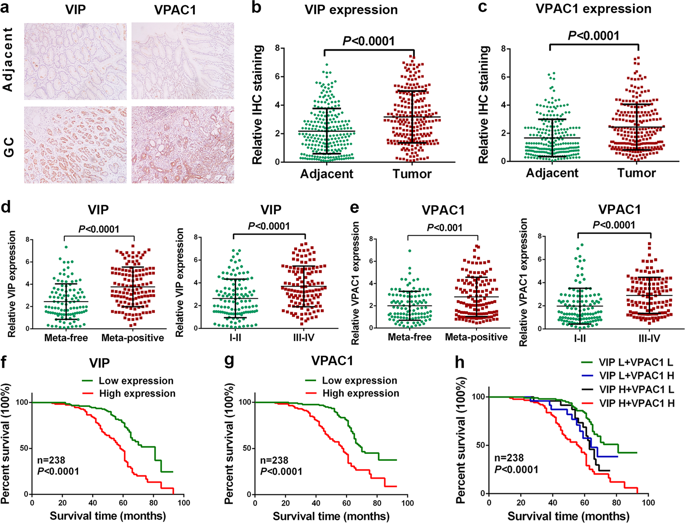
Although VPAC1 and its ligand vasoactive intestinal peptide (VIP) are important in gastrointestinal physiology, their involvements in progression of gastrointestinal tumor have not been explored. Here, we found that higher expression of VIP/VPAC1 was observed in gastric cancer compared to the adjacent normal tissues. The increased expression of VIP/VPAC1 in gastric cancer correlated positively with invasion, tumor stage, lymph node, distant metastases, and poor survival. Moreover, high expression of VIP and VPAC1, advanced tumor stage and distant metastasis were independent prognostic factors. VPAC1 activation by VIP markedly induced TRPV4-mediated Ca2+ entry, and eventually promoted gastric cancer progression in a Ca2+ signaling-dependent manner. Inhibition of VPAC1 and its signaling pathway could block the progressive responses. VPAC1/TRPV4/Ca2+ signaling in turn enhanced the expression and secretion of VIP in gastric cancer cells, enforcing a positive feedback regulation mechanism. Taken together, our study demonstrate that VPAC1 is significantly overexpressed in gastric cancer and VPAC1/TRPV4/Ca2+ signaling axis could enforce a positive feedback regulation in gastric cancer progression. VIP/VPAC1 may serve as potential prognostic markers and therapeutic targets for gastric cancer.
中文翻译:

VPAC1与TRPV4通道结合通过新的自分泌机制促进钙依赖性胃癌的进展
尽管VPAC1及其配体血管活性肠肽(VIP)在胃肠道生理中很重要,但尚未探讨它们在胃肠道肿瘤进展中的作用。在这里,我们发现与邻近的正常组织相比,在胃癌中观察到VIP / VPAC1的更高表达。胃癌中VIP / VPAC1表达的增加与浸润,肿瘤分期,淋巴结,远处转移和不良生存呈正相关。此外,VIP和VPAC1的高表达,晚期肿瘤分期和远处转移是独立的预后因素。VIP激活VPAC1会明显诱导TRPV4介导的Ca 2+进入,并最终促进Ca 2+中胃癌的进展依赖信号的方式。VPAC1及其信号通路的抑制作用可能会阻止进行性反应。VPAC1 / TRPV4 / Ca 2+信号转而增强了胃癌细胞中VIP的表达和分泌,从而增强了正反馈调节机制。综上所述,我们的研究表明VPAC1在胃癌中显着过表达,而VPAC1 / TRPV4 / Ca 2+信号轴可以在胃癌的进展过程中增强正反馈调节。VIP / VPAC1可能作为胃癌的潜在预后标志物和治疗靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号