Oncogene ( IF 6.9 ) Pub Date : 2019-01-28 , DOI: 10.1038/s41388-019-0698-5 Xiao-Lei Zhou , Chong-Yue Zhu , Zhi-Gang Wu , Xin Guo , Wei Zou
|
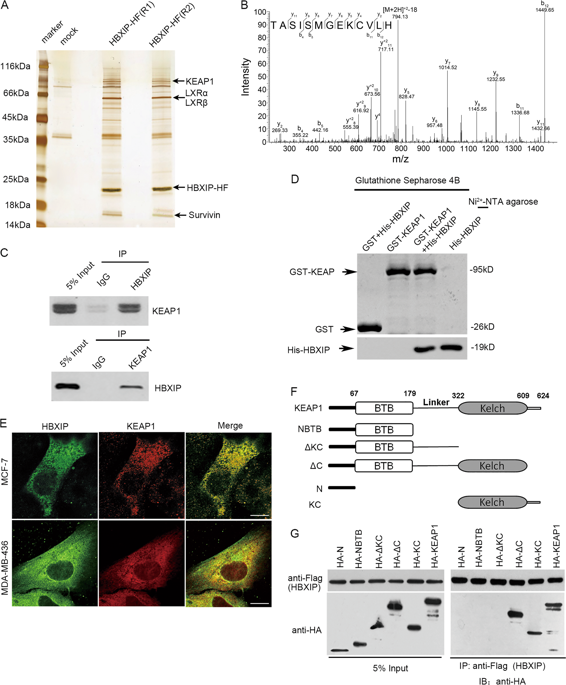
The nuclear factor E2-related factor 2 (NRF2)-Kelch-like ECH-associated protein 1 (KEAP1) signaling cascades is a key transcriptional pathway governing cellular oxidative stress and tumor development. Mammalian hepatitis B X-interacting protein (HBXIP) has critical roles in modulating cancer malignance and tumor progression. However, whether HBXIP interacts with KEAP1 and NRF2 is unclear. Here, we found that HBXIP can effectually compete with NRF2 for binding with KEAP1 protein via its highly conserved GLNLG motif. The HBXIP-mediated reduction in NRF2-KEAP1 complexes promotes NRF2 accumulation and nuclear entry, which facilities the activation of antioxidant response element (ARE)-dependent signaling cascades, thereby reducing the accumulation of endogenous cellular reactive oxygen species (ROS). We also found a strong positive correlation between HBXIP expression and NRF2 expression in breast cancer cells, tissue microarrays and clinical breast cancer tissues. Furthermore, this positive correlation was further confirmed via analysis of 1905 clinical cases of breast carcinoma provided by the cancer genomics database cBioPortal. Strikingly, disrupting the HBXIP-KEAP1 axis via mutating the GLNLG motif of HBXIP leads to potent inhibition of the malignancy of breast carcinoma both in vivo and in vitro. Our findings broaden our understanding of HBXIP as a modulation factor of cellular oxidative stress and address a novel regulatory mechanism governing redox homeostasis and the progression of breast carcinoma.
中文翻译:

癌蛋白HBXIP竞争性结合KEAP1以激活NRF2并增强乳腺癌细胞的生长和转移
核因子E2相关因子2(NRF2)-Kelch样ECH相关蛋白1(KEAP1)信号级联反应是控制细胞氧化应激和肿瘤发展的关键转录途径。哺乳动物乙型肝炎X相互作用蛋白(HBXIP)在调节癌症恶性程度和肿瘤进展中起关键作用。但是,HBXIP是否与KEAP1和NRF2交互尚不清楚。在这里,我们发现HBXIP可以通过其高度保守的GLNLG基序与NRF2有效竞争与KEAP1蛋白的结合。HBXIP介导的NRF2-KEAP1复合物的减少促进NRF2积累和核进入,这促进了抗氧化剂响应元件(ARE)依赖的信号级联的激活,从而减少了内源性细胞活性氧(ROS)的积累。我们还发现乳腺癌细胞,组织微阵列和临床乳腺癌组织中HBXIP表达与NRF2表达之间存在很强的正相关性。此外,通过癌症基因组学数据库cBioPortal提供的1905例乳腺癌临床病例分析,进一步证实了这种正相关性。引人注目的是,通过突变HBXIP的GLNLG基序来破坏HBXIP-KEAP1轴会导致体内和体外乳腺癌的恶性抑制。我们的发现拓宽了我们对HBXIP作为细胞氧化应激调节因子的理解,并探讨了控制氧化还原稳态和乳腺癌进展的新型调节机制。通过癌症基因组学数据库cBioPortal提供的1905例乳腺癌临床病例分析,进一步证实了这种正相关性。引人注目的是,通过突变HBXIP的GLNLG基序来破坏HBXIP-KEAP1轴会导致体内和体外乳腺癌的恶性抑制。我们的发现拓宽了我们对HBXIP作为细胞氧化应激调节因子的理解,并探讨了控制氧化还原稳态和乳腺癌进展的新型调节机制。通过癌症基因组学数据库cBioPortal提供的1905例乳腺癌临床病例分析,进一步证实了这种正相关性。引人注目的是,通过突变HBXIP的GLNLG基序来破坏HBXIP-KEAP1轴会导致体内和体外乳腺癌的恶性抑制。我们的发现拓宽了我们对HBXIP作为细胞氧化应激调节因子的理解,并探讨了控制氧化还原稳态和乳腺癌进展的新型调节机制。通过突变HBXIP的GLNLG基序破坏HBXIP-KEAP1轴导致体内和体外乳腺癌的恶性抑制。我们的发现拓宽了我们对HBXIP作为细胞氧化应激调节因子的理解,并探讨了控制氧化还原稳态和乳腺癌进展的新型调节机制。通过突变HBXIP的GLNLG基序破坏HBXIP-KEAP1轴导致体内和体外乳腺癌的恶性抑制。我们的发现拓宽了我们对HBXIP作为细胞氧化应激调节因子的理解,并探讨了控制氧化还原稳态和乳腺癌进展的新型调节机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号