npj Aging ( IF 4.1 ) Pub Date : 2016-04-07 , DOI: 10.1038/npjamd.2016.1 Elena M Vayndorf , Courtney Scerbak , Skyler Hunter , Jason R Neuswanger , Marton Toth , J Alex Parker , Christian Neri , Monica Driscoll , Barbara E Taylor
|
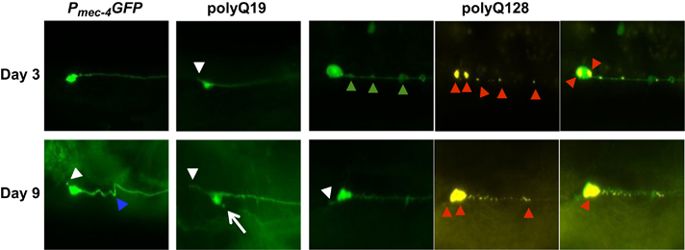
Understanding cellular outcomes, such as neuronal remodeling, that are common to both healthy and diseased aging brains is essential to the development of successful brain aging strategies. Here, we used Caenorhabdits elegans to investigate how the expression of proteotoxic triggers, such as polyglutamine (polyQ)-expanded huntingtin and silencing of proteostasis regulators, such as the ubiquitin–proteasome system (UPS) and protein clearance components, may impact the morphological remodeling of individual neurons as animals age. We examined the effects of disrupted proteostasis on the integrity of neuronal cytoarchitecture by imaging a transgenic C. elegans strain in which touch receptor neurons express the first 57 amino acids of the human huntingtin (Htt) gene with expanded polyQs (128Q) and by using neuron-targeted RNA interference in adult wild-type neurons to knockdown genes encoding proteins involved in proteostasis. We found that proteostatic challenges conferred by polyQ-expanded Htt and knockdown of specific genes involved in protein homeostasis can lead to morphological changes that are restricted to specific domains of specific neurons. The age-associated branching of PLM neurons is suppressed by N-ter polyQ-expanded Htt expression, whereas ALM neurons with polyQ-expanded Htt accumulate extended outgrowths and other soma abnormalities. Furthermore, knockdown of genes important for ubiquitin-mediated degradation, lysosomal function, and autophagy modulated these age-related morphological changes in otherwise normal neurons. Our results show that the expression of misfolded proteins in neurodegenerative disease such as Huntington’s disease modifies the morphological remodeling that is normally associated with neuronal aging. Our results also show that morphological remodeling of healthy neurons during aging can be regulated by the UPS and other proteostasis pathways. Collectively, our data highlight a model in which morphological remodeling during neuronal aging is strongly affected by disrupted proteostasis and expression of disease-associated, misfolded proteins such as human polyQ-Htt species.
中文翻译:

衰老过程中秀丽隐杆线虫神经元的形态重塑被受损的蛋白质稳态所改变
了解健康和患病的老化大脑共有的细胞结果,例如神经元重塑,对于制定成功的大脑老化策略至关重要。在这里,我们使用秀丽隐杆线虫来研究蛋白毒性触发物的表达,例如多聚谷氨酰胺 (polyQ) 扩增的亨廷顿蛋白和蛋白酶体调节剂的沉默,例如泛素-蛋白酶体系统 (UPS) 和蛋白质清除成分,可能如何影响形态重塑随着动物年龄的增长,单个神经元的变化。我们通过对触摸受体神经元表达人类亨廷顿蛋白的前 57 个氨基酸(Htt) 具有扩展的 polyQs (128Q) 的基因,并通过在成年野生型神经元中使用神经元靶向 RNA 干扰来敲低编码参与蛋白质稳态的蛋白质的基因。我们发现由 polyQ 扩展的 Htt 赋予的蛋白质稳态挑战和参与蛋白质稳态的特定基因的敲低可导致仅限于特定神经元特定域的形态变化。PLM 神经元的与年龄相关的分支被 N-ter polyQ 扩展的 Htt 表达抑制,而具有 polyQ 扩展的 Htt 的 ALM 神经元积累了扩展的生长和其他体细胞异常。此外,对泛素介导的降解、溶酶体功能和自噬很重要的基因的敲除调节了正常神经元中这些与年龄相关的形态学变化。我们的研究结果表明,在亨廷顿氏病等神经退行性疾病中错误折叠蛋白的表达改变了通常与神经元衰老相关的形态重塑。我们的研究结果还表明,衰老过程中健康神经元的形态重塑可以通过 UPS 和其他蛋白质稳态途径进行调节。总的来说,我们的数据突出了一个模型,其中神经元衰老过程中的形态重塑受到破坏的蛋白质稳态和疾病相关的错误折叠蛋白(如人类 polyQ-Htt 物种)的表达的强烈影响。我们的研究结果还表明,衰老过程中健康神经元的形态重塑可以通过 UPS 和其他蛋白质稳态途径进行调节。总的来说,我们的数据突出了一个模型,其中神经元衰老过程中的形态重塑受到破坏的蛋白质稳态和疾病相关的错误折叠蛋白(如人类 polyQ-Htt 物种)的表达的强烈影响。我们的研究结果还表明,衰老过程中健康神经元的形态重塑可以通过 UPS 和其他蛋白质稳态途径进行调节。总的来说,我们的数据突出了一个模型,其中神经元衰老过程中的形态重塑受到破坏的蛋白质稳态和疾病相关的错误折叠蛋白(如人类 polyQ-Htt 物种)的表达的强烈影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号